Abstract
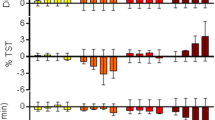
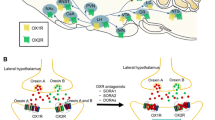
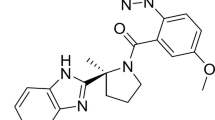
Suvorexant (Belsomra®), a first-in-class, orally active dual orexin-1 receptor and orexin-2 receptor antagonist, has been developed by Merck for the treatment of insomnia. Variations in the levels of the neuropeptides orexin A and orexin B have been linked to circadian rhythms and wakefulness. Orexin-producing neurons in the lateral hypothalamus regulate wakefulness by signalling through orexin receptors. Blockade of orexin receptors is known to promote sleep. Suvorexant was approved in the US in August 2014 for the treatment of adults with sleep onset and/or sleep maintenance insomnia. The drug is also preregistration in Japan, with approval submissions planned for other countries worldwide for this indication. This article summarizes the milestones in the development of suvorexant leading to this first approval for insomnia.
Similar content being viewed by others
References
Buyssee DJ. Insomnia. JAMA. 2013;309(7):706.
Mieda M, Sakurai T. Orexin (hypocretin) receptor agonists and antagonists for treatment of sleep disorders: rationale for development and current status. CNS Drugs. 2013;27(2):83–90.
US Food and Drug Administration. FDA approves new type of sleep drug, Belsomra [media release]. 13 August 2014.http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm409950.htm.
Merck & Co. BELSOMRA®(suvorexant) US prescribing information. 2014.http://www.merck.com/product/usa/pi_circulars/b/belsomra/belsomra_pi.pdf.Accessed 18 Aug 2014.
Merck & Co. Merck receives Complete Response Letter for suvorexant, Merck’s investigational medicine for insomnia [media release]. 1 July 2013.http://www.merck.com.
US Federal Register. Schedules of controlled substances: placement of suvorexant into schedule IV. 2014.https://www.federalregister.gov/articles/2014/08/28/2014-20515/schedules-of-controlled-substances-placement-of-suvorexant-into-schedule-iv?utm_campaign=subscription+mailing+list&utm_medium=email&utm_source=federalregister.gov.Accessed 10 Sep 2014.
Connor K, Budd K, Snavely D, et al. Efficacy and safety of suvorexant, an orexin receptor antagonist, in patients with primary insomnia: a 3-month phase 3 trial (trial #1) [abstract no. O324]. J Sleep Res. 2012;21(Suppl 1):97.
Ivgy-May N, Leibensperger H, Froman S, et al. Efficacy and safety of suvorexant, an orexin receptor antagonist, in patients with primary insomnia: a 3-month phase 3 trial (trial #2) [abstract no. P988]. J Sleep Res. 2012;21(Suppl 1):351–2.
Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461–71.
Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265–74.
Merck & Co. Merck announces full-year and fourth-quarter 2012 financial results. 2012.
Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant: a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1–2):52–61.
US Food and Drug Administration. Suvorexant Advisory Committee Meeting briefing document. 2013.http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/peripheralandcentralnervoussystemdrugsadvisorycommittee/ucm352970.pdf.Accessed 9 Sep 2014.
Sun H, Kennedy D, Lewis N, et al. The single dose pharmacokinetic (PK) and pharmacodynamic (PD) profiles of suvorexant (MK-4305), a dual orexin receptor antagonist, in healthy male subjects [abstract no. PI-59]. Clin Pharmacol Ther. 2012;91(Suppl 1):S29.
Sun H, Kennedy WP, Wilbraham D, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36(2):259–67.
Sun H, Yee KL, Khalilieh S, et al. Phase 1 safety, pharmacokinetics, and pharmacodynamics of the orexin receptor antagonist suvorexant administered with alcohol or paroxetine [abstract no. 0650]. Sleep. 2013;36(Suppl):A224.
Herring WJ, Ivgy-May N, Connor KM, et al. Effect of suvorexant, an orexin receptor antagonist, on patient-reported outcomes in patients with primary insomnia: integrated results from two phase-3 trials [abstract no. 0648]. Sleep. 2013;36(Suppl):A223.
Hisada S, Kikuchi M, Takahashi K, et al. Efficacy and safety of suvorexant, an orexin receptor antagonist, in patients with primary insomnia: a 3-month phase III study (P028) [abstract no. 0649]. Sleep. 2013;36(Suppl):A224.
Connor KM, Matzura-Wolfe D, Zhang Y, et al. Safety of suvorexant, an orexin receptor antagonist, in patients with primary insomnia: integrated phase 3 results [abstract no. 0647]. Sleep. 2013;36(Suppl):A223.
Vermeeren A, Vuurman E, Van Oers A, et al. Effects of suvorexant, an orexin receptor antagonist, on next day driving performance in healthy volunteers [abstract no. W11]. In: 51st Annual Meeting of the American College of Neuropsychopharmacology; 2–6 Dec 2012; Hollywood (FL).
Uemura N, McCrea J, Sun H, et al. Effects of supratherapeutic and therapeutic doses of the orexin receptor antagonist suvorexant on respiration during sleep in healthy subjects [abstract no. 0645]. Sleep. 2013;36(Suppl):A222.
Troyer M, Uemura N, McCrea J, et al. Respiratory safety of the orexin receptor antagonist suvorexant during sleep in patients with compromised respiratory function and in healthy subjects [abstract no. P5.293]. Neurology. 2014;82(10 Suppl 1).
Disclosure
The preparation of this report was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. L. P. H. Yang is a contracted employee of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from theAdis R&D Insightdrug pipeline database.Adis R&D Insighttracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch.
Rights and permissions
About this article
Cite this article
Yang, L.P.H. Suvorexant: First Global Approval. Drugs74,1817–1822 (2014). https://doi.org/10.1007/s40265-014-0294-5
Published:
Issue Date:
DOI:https://doi.org/10.1007/s40265-014-0294-5