Abstract
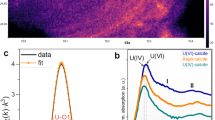
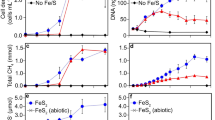
REDUCTION of the soluble, oxidized form of uranium, U(VI), to insoluble U(IV) is an important mechanism for the immobilization of uranium in aquatic sediments and for the formation of some uranium ores1–10.U(VI) reduction has generally been regarded as an abiological reaction in which sulphide, molecular hydrogen or organic compounds function as the reductant1,2,5,11.Microbial involvement in U(VI) reduction has been considered to be limited to indirect effects, such as microbial metabolism providing the reduced compounds for abiological U(VI) reduction and microbial cell walls providing a surface to stimulate abiological U(VI) reduction1,12,13.We report here, however, that dissimilatory Fe(III)-reducing microorganisms can obtain energy for growth by electron transport to U(VI). This novel form of microbial metabolism can be much faster than commonly cited abiological mechanisms for U(VI) reduction. Not only do these findings expand the known potential terminal electron acceptors for microbial energy transduction, they offer a likely explanation for the deposition of uranium in aquatic sediments and aquifers, and suggest a method for biological remediation of environments contaminated with uranium.
This is a preview of subscription content,access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Jensen, M. L.Econ. Geol.53,598–616 (1958).
Hostetler, P. B. & Garrels, R. M.Econ. Geol.57,137–167 (1962).
Turekian, K. K. & Bertine, K. K.Nature229,250–251 (1971).
Bonatti, E., Fisher. D. E., Joensuu, O. & Rydell, H. S.Geochim. cosmochim. Acta35,189–201 (1971).
Langmuir, D.Geochim. cosmochim. Acta42,547–569 (1978).
Kadko, D.Earth planet. Sci. Lett.51,115–131 (1980).
Colley, S. & Thomson, J.Geochim. cosmochim. Acta49,2339–2348 (1985).
Cochran, J. K., Carey, A. E., Sholkovitz, E. R. & Surprenant, L. D.Geochim. cosmochim. Acta50,663–680 (1986).
Anderson, R. F., LeHuray, A. P., Fleisher, M. Q. & Murray, J. W.Geochim. cosmochim. Acta53,2205–2213 (1989).
Wallace, H. E.et al.Geochim. cosmochim. Acta52,1557–1569 (1988).
Maynard, J. B.Geochemistry of Sedimentary Ore Deposits(Springer, New York, 1983).
Taylor, G. H. inBiogeochemical Cycling of Mineral-Forming Elements(eds Trudinger, P. A. & Swaine, D. J.) 485–514 (Elsevier, New York, 1979).
Mohagheghi, A., Updegraff, D. M. & Goldhaber, M. B.Geomicrobiol. J.4,153–173 (1985).
Ehrlich, H. L.Geomicrobiology(Marcel Dekker, New York, 1990).
Ghiorse, W. C. inBiology of Anaerobic Microorganisms(ed. Zehnder, A. J. B.) 305–331 (Wiley, New York, 1988).
Lovley, D. R., Stolz, J. F., Nord, G. L. & Phillips, E. J. P.Nature330,252–254 (1987).
Lovley, D. R. & Phillips, E. J. P.Appl. environ. Microbiol.54,1471–1480 (1988).
Lovley, D. R., Phillips, E. J. P. & Lonergan, D. J.Appl. environ. Microbiol.55,700–706 (1989).
Myers, C. R. & Nealson, K. H.Science240,1319–1321 (1988).
Woolfolk, C. A. & Whiteley, H. R.J. Bacteriol.84,647–658 (1962).
Lovley, D. R. & Phillips, E. J. P.Appl. environ. Microbiol.53,2636–2641 (1987).
Anderson, R. F., Fleisher, M. Q. & LeHuray, A. P.Geochim. cosmochim. Acta53,2215–2224 (1989).
Anderson, R. F.Uranium3,145–164 (1987).
Breger, I. A. inFormation of Uranium Ore Deposits99–124 (International Atomic Energy Agency, Vienna, 1974).
Nakashima, S., Disnar, J. R., Perruchot, A. & Trichet, J.Geochim. cosmochim. Acta48,2321–2329 (1984).
Hoffman, B. A.Chem. Geol.81,55–81 (1990).
Harrison, R. K.G.B. geol. Surv. Bull.52,1–26 (1975).
Lovley, D. R., Chapelle, F. H. & Phillips, E. J. P.Geology18,954–957 (1990).
Lovley, D. R.et al.Nature339,297–299 (1989).
Lovley, D. R. & Lonergan, D. J.Appl. environ. Microbiol.56,1858–1864 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lovley, D., Phillips, E., Gorby, Y.et al.Microbial reduction of uranium. Nature350,413–416 (1991). https://doi.org/10.1038/350413a0
Received:
Accepted:
Issue Date:
DOI:https://doi.org/10.1038/350413a0