Abstract
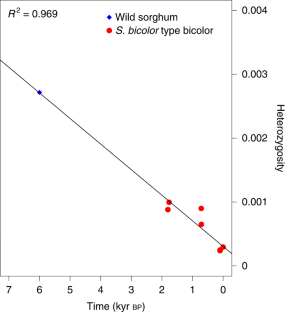
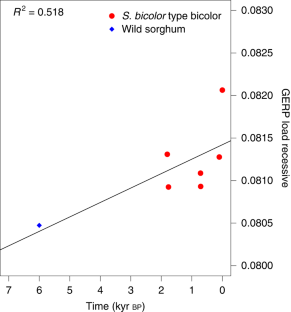
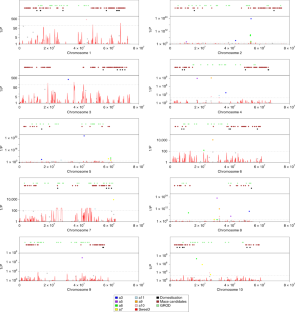
The evolution of domesticated cereals was a complex interaction of shifting selection pressures and repeated episodes of introgression. Genomes of archaeological crops have the potential to reveal these dynamics without being obscured by recent breeding or introgression. We report a temporal series of archaeogenomes of the crop sorghum (Sorghum bicolor) from a single locality in Egyptian Nubia. These data indicate no evidence for the effects of a domestication bottleneck, but instead reveal a steady decline in genetic diversity over time coupled with an accumulating mutation load. Dynamic selection pressures acted sequentially to shape architectural and nutritional domestication traits and to facilitate adaptation to the local environment. Later introgression between sorghum races allowed the exchange of adaptive traits and achieved mutual genomic rescue through an ameliorated mutation load. These results reveal a model of domestication in which genomic adaptation and deterioration were not focused on the initial stages of domestication but occurred throughout the history of cultivation.
This is a preview of subscription content,access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 €/ 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
Sequence data were deposited in the European Molecular Biology Laboratory European Bioinformatics Institute (project codePRJEB24962).
Code availability
The serial founder event simulation was executed using the program founderv6.pl available for download at:https://warwick.ac.uk/fac/sci/lifesci/research/archaeobotany/downloads/founderv6.
References
Larson, G. et al. Current perspectives and the future of domestication studies.Proc. Natl Acad. Sci. USA111,6139–6146 (2014).
Purugganan, M. D. & Fuller, D. Q. The nature of selection during plant domestication.Nature457,843–848 (2009).
Fuller, D. Q. et al. Convergent evolution and parallelism in plant domestication revealed by an expanding archaeological record.Proc. Natl Acad. Sci. USA111,6147–6152 (2014).
Allaby, R. G., Stevens, S., Lucas, L., Maeda, O. & Fuller, D. Q. Geographic mosaics and changing rates of cereal domestication.Phil. Trans. R. Soc. B372,20160429 (2017).
Poets, A. M., Fang, Z., Clegg, M. T. & Morell, P. L. Barley landraces are characterized by geographically heterogeneous genomic origins.Genome Biol.16,173 (2015).
Hardigan, M. A. et al. Genome diversity of tuber-bearing solanum uncovers complex evolutionary history and targets of domestication in the cultivated potato.Proc. Natl Acad. Sci. USA114,E9999–E10008 (2017).
Hufford, M. et al. The genomic signature of crop-wild introgression in maize.PLoS Genet.9,e1003477 (2013).
The World Sorghum and Millet Economies: Facts, Trends and Outlook(FAO and ICRISAT, 1996)..
Winchell, F., Stevens, C. J., Murphy, C., Champion, L. & Fuller, D. Q. Evidence for sorghum domestication in fourth millennium BC eastern Sudan: spikelet morphology from ceramic impressions of the Butana group.Curr. Anthropol.58,673–683 (2017).
Doggett, H.Sorghum2nd edn (Longman, 1988).
Brown, P. J., Myles, S. & Kresowich, S. Genetic support for a phenotype-based racial classification in sorghum.Crop Sci.51,224–230 (2011).
Morris, G. et al. Population genomic and genome-wide association studies of agroclimatic traits in sorghum.Proc. Natl Acad. Sci. USA110,453–458 (2013).
Fuller, D. Q. & Stevens, C. J. inPlants and People in Africa’s Past: Progress in African Archaeobotany(eds Mercuri, A. M. et al.) 427–452 (Springer, 2017).
Clapham, A. J. & Rowley-Conwy, P. A. inFields of Change: Progress in African Archaeobotany(ed. Cappers, R.) 157–164 (Barkhuis & Groningen University Library, 2007).
de Wet, J. M. L., Harlan, J. R. & Price, E. G. inOrigins of African Plant Domestication(eds Harlan, J. R. et al.) 453–463 (Mouton Press, 1976).
Harlan, J. R. & Stemler, A. B. L. inOrigins of African Plant Domestication(eds Harlan, J. R. et al.) 465–478 (Mouton Press, 1976).
Ohadi, S., Hodnett, G., Rooney, W. & Bagavathiannan, M. Gene flow and its consequences inSorghumspp.Crit. Rev. Plant Sci.36,367–385 (2017).
Meyer, R. & Purugganan, M. Evolution of crop species: genetics of domestication and diversification.Nat. Rev. Genet.14,840–852 (2013).
Li, H. & Durbin, R. Inference of human population history from individual whole-genome sequences.Nature475,493–496 (2011).
Wang, L. et al. The interplay of demography and selection during maize domestication and expansion.Genome Biol.18,215 (2017).
Meyer, R. S. et al. Domestication history and geographical adaptation inferred from a SNP map of African rice.Nat. Genet.48,1083–1088 (2016).
Mazet, O., Rodríguez, W., Grusea, S., Boitard, S. & Chikhi, L. On the importance of being structured: instantaneous coalescence rates and human evolution—lessons for ancestral population size inference.Heredity (Edinb.)116,362–371 (2016).
Orozco-terWengel, P. The devil is in the details: the effect of population structure on demographic inference.Heredity (Edinb.)116,349–350 (2016).
Renault, S. & Rieseberg, L. The accumulation of deleterious mutations as a consequence of domestication and improvement in sunflower and other Compositae crops.Mol. Biol. Evol.32,2273–2283 (2015).
Liu, Q., Zhou, Y., Morrell, P. L. & Gaut, B. S. Deleterious variants in Asian rice and the potential cost of domestication.Mol. Biol. Evol.34,908–924 (2017).
Cooper, G. M. et al. Distribution and intensity of constraint in mammalian genomic sequence.Genome Res.15,901–913 (2005).
Smith, O. et al. Genomic methylation patterns in archaeological barley show de-methylation as a time-dependent diagenetic process.Sci. Rep.4,5559 (2014).
Pavlidis, P., Živkovic, D., Stamatakis, A. & Alachiotis, N. SweeD: likelihood-based detection of selective sweeps in thousands of genomes.Mol. Biol. Evol.30,2224–2234 (2013).
Hudson, M., Ringli, C., Boylan, M. T. & Quail, P. H. TheFAR1locus encodes a novel nuclear protein specific to phytochrome a signaling.Genes Dev.13,2017–2027 (1999).
Zhu, Q. H., Ramm, K., Shivakkumar, R., Dennis, E. S. & Upadhyahya, N. M. TheANTHER INDEHISCENCE1gene encoding a single MYB domain protein is involved in anther development in rice.Plant Physiol.135,1514–1525 (2004).
Martin, S., Davey, J. & Jiggins, C. Evaluating the use of ABBA–BABA statistics to locate introgressed loci.Mol. Biol. Evol.32,244–257 (2014).
Mace, E. S. et al. Whole genome sequencing reveals untapped genetic potential in Africa’s indigenous cereal crop sorghum.Nat. Commun.4,2320 (2013).
Thurber, C. S., Ma, J. M., Higgins, R. H. & Brown, P. J. Retrospective genomic analysis of sorghum adaptation to temperate-zone grain production.Genome Biol.14,R68 (2013).
DeGiorgio, M., Jakobsson, M. & Rosenberg, N. Explaining worldwide patterns of human genetic variation using a coalescent-based serial founder model of migration outward from Africa.Proc. Natl Acad. Sci. USA106,16057–16062 (2009).
Alvarez, N. et al. Farmers’ practices, metapopulation dynamics, and conservation of agricultural biodiversity on-farm: a case study of sorghum among the Duupa in sub-sahelian Cameroon.Biol. Conserv.121,533–543 (2005).
Westengen, O. T. et al. Ethnolinguistic structuring of sorghum genetic diversity in Africa and the role of local seed systems.Proc. Natl Acad. Sci. USA111,14100–14105 (2014).
Allaby, R. G., Ware, R. & Kistler, L. A re-evaluation of the domestication bottleneck from archaeogenomic evidence.Evol. Appl.12,29–37 (2018).
Kremling, K. A. et al. Dysregulation of expression correlates with rare-allele burden and fitness loss in maize.Nature555,520–523 (2018).
Rogers & Slatkin Excess defects in a woolly mammoth on Wrangel Island.PLoS Genet.13,e1006601 (2017).
Shennan, S. et al. Regional population collapse followed initial agricultural booms in mid-Holocene Europe.Nat. Commun.4,2486 (2013).
Allaby, R. G., Kitchen, J. L. & Fuller, D. Q. Surprisingly low limits of selection in plant domestication.Evol. Bioinform. Online11,41–51 (2016).
Alexander, J. The Saharan divide in the Nile Valley: the evidence from Qasr Ibrim.Afr. Archaeol. Rev.6,73–90 (1988).
Rose, P. inThe Encyclopedia of Ancient History(eds Bagnall, R. S. et al.) 5695–5697 (Wiley, 2013).
Palmer, S. A., Moore, J. D., Clapham, A. J., Rose, P. & Allaby, R. G. Archaeogenetic evidence of ancient Nubian barley evolution from six to two-row indicates local adaptation.PLoS ONE4,e6301 (2009).
Palmer, S. A. et al. Archaeogenomic evidence of punctuated genome evolution inGossypium.Mol. Biol. Evol.29,2031–2038 (2012).
O’Donoghue, K., Clapham, A., Evershed, R. P. & Brown, T. A. Remarkable preservation of biomolecules in ancient radish seeds.Proc. Biol. Sci.263,541–547 (1996).
Alexander, J. & Driskell, B. Qasr Ibrim 1984.J. Egypt. Archaeol.71,12–26 (1985).
Rowley-Conwy, P. Nubia AD 0-550 and the ‘Islamic’ agricultural revolution: preliminary botanical evidence from Qasr Ibrim, Egyptian Nubia.Archeol. du Nil Moyen3,131–138 (1989).
Clapham, A. & Rowley-Conwy, P. inFrom Foragers to Farmers: Papers in Honour of Gordon C. Hillman(eds Fairbairn, A. S. & Weiss, E.) 244–253 (Oxbow Books, 2009).
Adams, W. Y.Ceramic Industries of Medieval Nubia(Univ. Kentucky Press, 1986).
Hanghøj, K. et al. Fast, accurate and automatic ancient nucleosome and methylation maps with epiPALEOMIX.Mol. Biol. Evol.33,3248–3298 (2016).
Paterson, A. H. et al. TheSorghum bicolorgenome and the diversification of grasses.Nature457,551–556 (2009).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2.Nat. Methods9,357–359 (2012).
Li, H. et al. The sequence alignment/map (SAM) format and SAMtools.Bioinformatics25,2078–2079 (2009).
Bouyer, D. et al. DNA methylation dynamics during early plant life.Genome Biol.18,179 (2017).
Clark, R. M., Tavare, S. & Doebley, J. Estimating a nucleotide substitution rate for maize from polymorphism at a major domestication locus.Mol. Biol. Evol.22,2304–2312 (2005).
Van der Auwera, G. A. et al. From FastQ data to high-confidence variant calls: the genome analysis toolkit best practices pipeline.Curr. Protoc. Bioinformatics43,11.10.1–11.10.33 (2013).
da Fonseca, R. R. et al. The origin and evolution of maize in the southwestern United States.Nat. Plants1,14003 (2015).
Koslov, A. M., Aberer, A. & Alexandros, S. ExaML version 3: a tool for phylogenomic analysis on supercomputers.Bioinformatics31,2577–2579 (2015).
Felsenstein, J. Evolutionary trees from DNA sequences: a maximum likelihood approach.J. Mol. Evol.17,368–376 (1981).
Stamatakis, A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models.Bioinformatics22,2688–2690 (2006).
R:A Language and Environment for Statistical Computing(R Core Team, 2017);https://www.r-project.org
Pfeifer, B., Wittelsbürger, U., Ramos-Onsins, S. & Lercher, M. PopGenome: an efficient Swiss army knife for population genomic analyses in R.Mol. Biol. Evol.31,1929–1936 (2014).
Wolfe, K. H., Sharpe, P. M. & Li, W. H. Rates of synonymous substitution in plant nuclear genes.J. Mol. Evol.29,208–211 (1989).
Acknowledgements
The authors thank M. Nesbitt for permitting the use of herbaria material from Kew Gardens. O.S., W.V.N., G.B. and R.G.A. were supported by the NERC (NE/L006847/1), and L.K. was also supported by NERC (NE/L012030/1). The work by C.S. and D.Q.F. with archaeobotanical materials was supported by a European Research Council grant (no. 323842).
Author information
Authors and Affiliations
Contributions
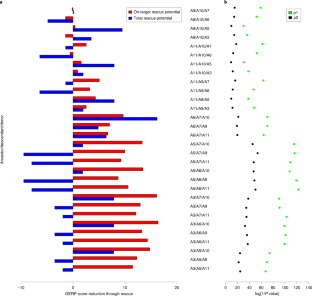
R.G.A. conceived and designed the project. R.G.A., O.S., R.W. and L.K. wrote the manuscript. R.G.A., O.S., W.V.N., L.K., E.M., R.W., G.B. and S.S. interpreted the genomic data; C.S., A.C. and D.Q.F. interpreted the archaeological data. O.S. performed DNA extractions; genome sequencing; baseline bioinformatics, including mapping heterozygosity calls across genomes and specific domestication genes; methylation analysis; and PSMC analysis. P.R., A.C. and D.Q.F. supplied archaobotanical material; C.S., A.C. and D.Q.F. selected archaobotanical material. W.V.N. performed phylogenetic, SweeD and linear regression analyses. L.K. and R.G.A. performed GERP analyses. E.M. and D.J. performed principal component analysis and provided access to datasets. R.G.A. performed heterozygosity, heterozygosity gradient and population simulation analyses. R.W. performed linear regression analysis. S.S. performed D statistic analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information:Nature Plantsthanks Terence Brown, Xuehui Huang and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note:Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–15, Supplementary Figures 1–18 and Supplementary Video Legend.
Supplementary Video
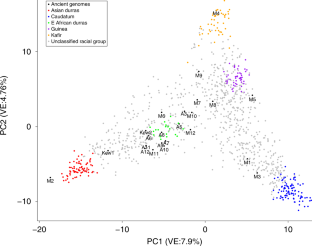
A three-dimensional file showing principal coordinate analysis of 1,894 SNPs from 23 genomes in this study and 1,046Sorghumlines described in ref. 25.
Rights and permissions
About this article
Cite this article
Smith, O., Nicholson, W.V., Kistler, L.et al.A domestication history of dynamic adaptation and genomic deterioration inSorghum. Nat. Plants5,369–379 (2019). https://doi.org/10.1038/s41477-019-0397-9
Received:
Accepted:
Published:
Issue Date:
DOI:https://doi.org/10.1038/s41477-019-0397-9
This article is cited by
-
Ethnobotanical studies on rice landraces under on-farm conservation in Xishuangbanna of Yunnan Province, China
Journal of Ethnobiology and Ethnomedicine(2024)
-
Are cereal grasses a single genetic system?
Nature Plants(2024)
-
Unveiling the Genomic Symphony: Identification Cultivar-Specific Genes and Enhanced Insights on Sweet Sorghum Genomes Through Comprehensive superTranscriptomic Analysis
Journal of Molecular Evolution(2024)
-
The evolution and expansion of RWP-RK gene family improve the heat adaptability of elephant grass (Pennisetum purpureum Schum.)
BMC Genomics(2023)
-
Domestication of the Amazonian fruit tree cupuaçu may have stretched over the past 8000 years
Communications Earth & Environment(2023)