Over the past decade, atypical (or second-generation) antipsychotics have been increasingly used in the treatment of schizophrenia in preference to ‘conventional’ typical (first-generation) drugs. However, meta-analyses of clinical trials in participants with chronic schizophrenia have suggested a limited advantage of the newer agents in terms of efficacy Reference Geddes, Freemantle, Harrison and Bebbington1–Reference Leucht, Corves, Arbter, Engel, Li and Davis4 and two recent large trials failed to find a difference between these two classes of antipsychotics. Reference Lieberman, Stroup, McEvoy, Swartz, Rosenheck and Perkins5,Reference Jones, Barnes, Davies, Dunn, Lloyd and Hayhurst6 Furthermore, economic analyses have raised doubts about the cost-effectiveness of the newer drugs. Reference Rosenheck, Leslie, Sindelar, Miller, Lin and Stroup7,Reference Davies, Lewis, Jones, Barnes, Gaughran and Hayhurst8 Cost is an important factor for low- and middle-income countries, particularly if, as suggested by a previous meta-analysis, Reference Large, Farooq, Nielssen and Slade9 the cost of treatment could partly explain the inverse association between longer duration of untreated psychosis and per capita income. In this context, it has been suggested that typical antipsychotics are as useful as atypicals in the treatment of schizophrenia. Reference Lewis and Lieberman10
Individuals presenting with a first episode of schizophrenia differ from those in whom the disorder is well established in that a higher proportion show a good symptomatic response. Reference Robinson, Woerner, Alvir, Geisler, Koreen and Sheitman11 Moreover, the dose of antipsychotics required to achieve symptomatic remission is usually lower than in individuals with chronic schizoprhenia, Reference Oosthuizen, Emsley, Jadri Turner and Keyter12 and those with a first episode are more susceptible to extrapyramidal side-effects. Reference Sanger, Lieberman, Tohen, Grundy, Beasley and Tollefson13 Their younger age also puts them at risk of longer exposure to the potential metabolic complications of newer antipsychotics. Reference Smith, Hopkins, Peveler, Holt, Woodward and Ismail14 Avoiding adverse effects when individuals first start treatment is particularly important as it may colour their attitude to medication and psychiatric treatment more generally thereafter. Antipsychotics with a benign side-effect profile thus offer an advantage in this phase of schizophrenia. This is particularly important considering the high rates of non-adherence to medication in this population, reported to be as high as 50%. Reference Cotton, Lambert, Schimmelmann, Foley, Morley, McGorry and Conus15 Regarding the question whether atypical or typical antipsychotics are better for this population a Cochrane meta-analysis from 2003 found that there was not enough evidence at that time to make recommendations. Reference Rummel, Hamann, Kissling and Leucht16
In the UK, a recent update to the National Institute for Health and Clinical Excellence (NICE) guideline has moved away from the previous recommendation of atypical antipsychotics as a first-line treatment in people presenting with a first episode of psychosis. 17 We performed a meta-analysis to look at the efficacy and side-effects of atypical versus typical antipsychotics in the treatment of the early phase of psychosis.
Method
We included randomised controlled trials comparing atypical and typical antipsychotics in individuals in the early phase of psychosis. To be included in the meta-analysis, studies had to use a validated diagnostic system and explicitly define whether their participants were antipsychotic naive, experiencing a first episode of psychosis or were in the early phase of psychosis. We expected to find varying definitions of first episode and early psychosis, and therefore we accepted any definitions used and modelled the effects of the different duration of illness in the analyses. We excluded studies that recruited only participants with an affective psychosis, or individuals younger than 13 or older than 65 years of age. Studies needed to report at least one of the following outcomes: adherence with antipsychotic medication, symptom scales, weight or any validated measure of extrapyramidal side-effects.
In line with a recent long-term study, Reference Lieberman, Stroup, McEvoy, Swartz, Rosenheck and Perkins5 we selected ‘discontinuation for any cause’ at 12 months of the assigned antipsychotic (or when this was not reported, between 6–24 months), which includes drug efficacy and tolerance, as our primary measure of effectiveness. We also selected a measure of medication efficacy consisting of symptom scores at 12 weeks (or when this was not reported, between 6–18 weeks), as this is the time point at which a previous study showed that 90% of individuals with a first episode who responded to medication achieved symptomatic remission. Reference Emsley, Rabinowitz and Medori18 When more than one symptom scale was reported, we chose to use the Positive and Negative Syndrome Scale (PANSS) first, then the Brief Psychiatric Rating Scale (BPRS) and then any other validated scale. Two major groups of side-effects were assessed: weight gain and extrapyramidal side-effects, using the last reported observation of each study. When studies reported more than one arm of atypical or typical antipsychotics, effect sizes and variances of the different drugs were pooled together per group using number of participants as a weight factor.
Pertinent randomised controlled trials were identified using an electronic and a hand-based search. A computer-based search was performed using the following databases: MEDLINE (1966 to 20th January 2009), EMBASE (1980 to January Week 3 2009), PsycINFO (1806 to January Week 2 2009) and Cochrane CENTRAL (The Cochrane Library 2008, Issue 4). No language constraints were applied. Subject headings including Psychosis, Schizophrenia, and Atypical Antipsychotic Agent were used as well as text words such as ‘first episode’, ‘never medicated’, ‘naïve’ and names of atypical antipsychotics. Details of the search strategy can be found in the online supplement. Two of the authors (N.A.C. and M.C.) independently reviewed all the identified abstracts from the electronic search, selected the studies included and extracted the data. Any conflicts were discussed with a third reviewer (P.P.). References of the identified studies and published reviews on pharmacological management of first-episode psychosis were also searched. Reference Rummel, Hamann, Kissling and Leucht16,Reference Robinson, Woerner, Delman and Kane19 One of the authors (N.A.C.) undertook to hand-search data of published abstracts of the 3rd to 5th Conference on Early Psychosis and 8th to 14th Biennial Winter Workshop on Schizophrenia to complement the electronic search. Authors were contacted if data were missing.
As we anticipated different definitions of early psychosis or first-episode psychosis we expected to find significant clinical heterogeneity among the studies and therefore decided that using a random-effects analysis would be appropriate. Reference DerSimonian and Laird20 When looking at adherence rates for antipsychotic medication, we preferred to use odds ratios instead of risk ratios in spite of their more difficult interpretation. This allowed us to avoid giving too much weight to trials with high event rates. Reference Egger and Davey Smith21 For continuous variables, weighted means or standardised mean differences using Hedges'gwere used. The latter was used when more than one scale was reported in the outcomes. Heterogeneity was explored using χ2.I 2is another measure of heterogeneity that refers to the proportion of the variance explained by the between-trial variance. Due to the easiness in the interpretation, it is also reported in this study. We used meta-regression to look at potential confounders in our summary measure. Knowing that we would find a limited amount of trials to include in each comparison, we only performed the regression if the variable being controlled for was reported in all the included studies. We used meta-regressions to look at the effects of the dose of typical antipsychotic used and the proportion of antipsychotic-naive participants included. The European First-Episode Schizophrenia Trial (EUFEST) suggested that masking status might cause a bias in discontinuation rates (e.g. unmasked clinicians might discontinue typical drugs sooner than atypicals if complications arose). Reference Kahn, Fleischhacker, Boter, Davidson, Vergouwe and Keet22 Masking status was therefore also included in the model for discontinuation rates. For the side-effects analysis, we included the assessment point in time. Given the known difference in side-effect profiles for some atypicals, we also included in the regression the proportion of participants taking olanzapine or clozapine when looking at weight gain, and amisulpride or risperidone when looking at extrapyramidal symptoms. In order to avoid spurious results by overfitting the data (e.g. when more than one factor was reported in all the studies), we opted for a repeated univariate meta-regression rather than a multiple regression approach. Results of these meta-regressions should be considered exploratory because of the multiple comparisons performed. Publication bias was explored using Egger's test. Reference Egger, Davey Smith, Schneider and Minder23 All analyses were done using STATA 10.0 running in Windows XP.
Results
Combined searches of the four databases yielded 1053 references. In total 105 articles were retrieved for further assessment from which 14 different randomised controlled trials were identified. Two further trials were identified in the manual search, one of which could not be included because of a lack of data (the trial looked at ziprasidone versus haloperidol and was published as a conference abstract). As a result, we included 15 randomised controlled trials that recruited a total of 2522 patients.Figure 1summarises the study selection and exclusion process.

Fig. 1Flowchart of study selection. RCT, randomised controlled study.
Risperidone was used in nine studies as the atypical antipsychotic, Reference Emsley24–Reference Saddichha, Manjunatha, Ameen and Akhtar32 olanzapine was used in seven trials, Reference Sanger, Lieberman, Tohen, Grundy, Beasley and Tollefson13,Reference Kahn, Fleischhacker, Boter, Davidson, Vergouwe and Keet22,Reference Wu, Zhao, Liu, Zhai, Guo and Guo27,Reference Crespo-Facorro, Pérez-Iglesias, Ramirez-Bonilla, Martínez-García, Llorca and Luis Vázquez-Barquero28,Reference Saddichha, Manjunatha, Ameen and Akhtar32–Reference Lieberman, Tollefson, Tohen, Green, Gur and Kahn34 and two studies used quetiapine Reference Kahn, Fleischhacker, Boter, Davidson, Vergouwe and Keet22,Reference Bustillo, Rowland, Jung, Brooks, Qualls and Hammond35 and clozapine. Reference Wu, Zhao, Liu, Zhai, Guo and Guo27,Reference Lieberman, Phillips, Gu, Stroup, Zhang and Kong36 Amisulpride and ziprasidone were used in one study. Reference Kahn, Fleischhacker, Boter, Davidson, Vergouwe and Keet22 Twelve of the fifteen studies used haloperidol as the first-generation antipsychotic. The other three studies used chlorpromazine, Reference Lieberman, Phillips, Gu, Stroup, Zhang and Kong36 oral zuclopenthixol Reference Mackeprang, Kristiansen and Glenthøj25 and sulpiride. Reference Wu, Zhao, Liu, Zhai, Guo and Guo27 All but one study Reference Saddichha, Manjunatha, Ameen and Akhtar32 reported using low doses of typical antipsychotic, below the usually described cut-off point of 12 mg of haloperidol or equivalent. Reference Geddes, Freemantle, Harrison and Bebbington1,Reference Leucht, Corves, Arbter, Engel, Li and Davis4 Eight studies reported using doses lower than haloperidol 5 mg, Reference Kahn, Fleischhacker, Boter, Davidson, Vergouwe and Keet22,Reference Mackeprang, Kristiansen and Glenthøj25–Reference Wu, Zhao, Liu, Zhai, Guo and Guo27,Reference Brewer, Yücel, Harrison, McGorry, Olver and Egan29,Reference Gaebel, Riesbeck, Wölwer, Klimke, Eickhoff and von Wilmsdorff30,Reference de Haan, van Bruggen, Lavalaye, Booij, Dingemans and Linszen33,Reference Lieberman, Tollefson, Tohen, Green, Gur and Kahn34,accounting for more than two-thirds of the total sample of participants treated with typical antipsychotics included in this review. Characteristics of the included trials and selected references are shown in online Table DS1.
Seven studies reported long-term data of discontinuation rates suitable for pooling, with a total of 1823 participants. Studies used different definitions of discontinuation, but most reported that participants were considered as discontinuing the drug due to side-effects or lack of response among other reasons. Only one study considered discontinuation rates as their primary outcome and explicitly defined how they measured it. Reference Kahn, Fleischhacker, Boter, Davidson, Vergouwe and Keet22 There was a non-significant greater proportion of individuals prescribed atypical antipsychotics who were adherent around 1 year (Fig. 2,odds ratio (OR) = 0.73,P= 0.22). On visual inspection, results appeared to differ between the different studies and not surprisingly, heterogeneity was significant as assessed with χ2(P<0.001). There is an outlier finding from a small study that used quetiapine, Reference Bustillo, Rowland, Jung, Brooks, Qualls and Hammond35 and excluding this study did not change the overall result (OR = 0.64,P= 0.09) and had little effect on the heterogeneity observed. Masking status was reported in all seven studies. A meta-regression including masking status as the independent variable and the odds ratio of each study as the dependent variable was non-significant. In other words, masking status was not a significant moderator of the effect sizes of the studies included.

Fig. 2Comparison of discontinuation rates among participants receiving first-v.second-generation antipsychotics.
P= 0.22. Heterogeneity χ2= 28.55 (d.f. = 6)P<0.001,I 2= 79.0%. EPGN, Early Psychosis Global Network; GRNS, German Research Network on Schizophrenia; EUFEST, European First-Episode Schizophrenia Trial.
For clarity purposes, the first author of the first published paper is used in Table DS1 in cases where more than one article reporting outcomes of the study has been included.
For the short-term symptomatic outcome, 12 trials were pooled with a total of 1949 participants. Most studies reported PANSS scores, although a few reported BPRS scores. Therefore standardised effect sizes were pooled. A small non-significant trend favouring atypical antipsychotics was found (s.d. = –0.1,P= 0.12) as shown inFig. 3.Heterogeneity was not statistically significant in the comparison according to χ2(P= 0.17). Since there was no significant heterogeneity found, we repeated the analysis with a fixed-effect approach as some authors have suggested, but the difference remained non-significant (s.d. = –0.08, 95% CI –0.17 to 0.02). We performed a meta-regression using the dose of atypical as a covariate of the effect size. There was a non-significant trend for comparisons that used higher doses of typical antipsychotics to report larger effect sizes that favoured atypicals (1 mg of haloperidol equivalent accounting for standardised mean difference (SMD) = 0.02 favouring atypicals,P= 0.09).

Fig. 3Comparisons of symptoms scales at short term (around 3 months) between the two groups.
Effect sizes were standardised using Hedges'gand pooled using a random-effects model. Non-significant difference favouring atypicals shown (P= 0.12). Heterogeneity χ2= 15.3 (d.f. = 11)P= 0.17,I 2= 28%. EPGN, Early Psychosis Global Network; GRNS, German Research Network on Schizophrenia; EUFEST, European First-Episode Schizophrenia Trial.
For weight gain, a total of seven studies were pooled including 1444 participants. Pooling of the seven studies showed that individuals on atypical antipsychotics gained an extra 2.1 kg compared with those on typical antipsychotics (P= 0.04,Fig. 4). Heterogeneity was present according to χ2(P<0.001). One of the trials Reference Wu, Zhao, Liu, Zhai, Guo and Guo27,Reference Wu, Zhao, Zhai, Guo and Guo37 reported body max index (BMI) instead of weight. In order to include this outcome with the rest of the studies, participants' BMIs were transformed to kilograms assuming that everyone's height was 1.70 m (an assumption that probably decreased its variance). Excluding this study from the analysis had little effect on the results and only marginally decreased heterogeneity (I Reference Leucht, Wahlbeck, Hamann and Kissling2 decreased from 80 to 74%). Meta-regressions were performed using the following covariates: amount of time exposed, typical doses, and percentage of participants receiving olanzapine or clozapine. None of these factors reached statistical significance.
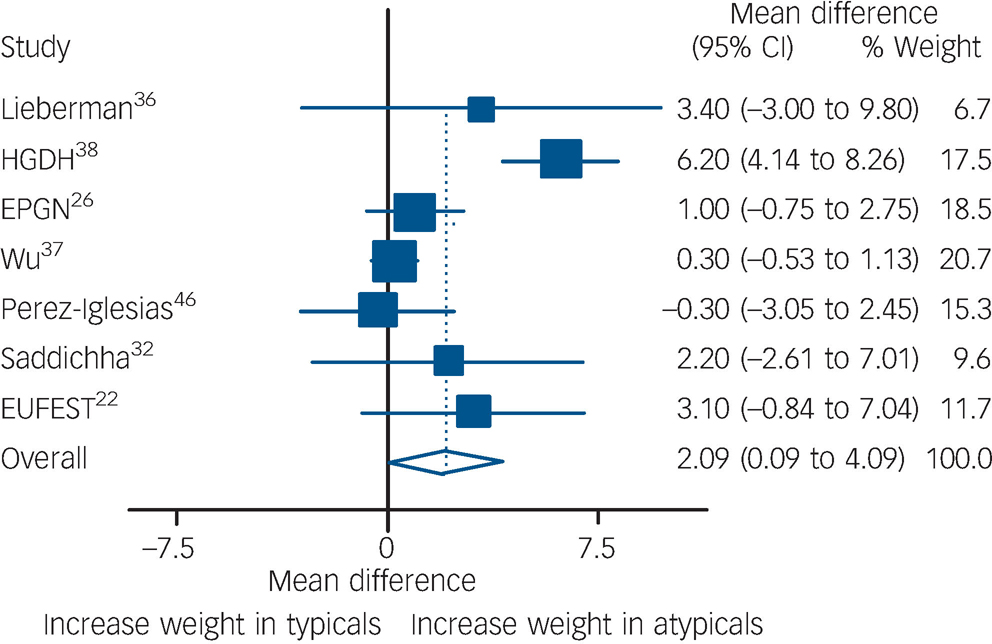
Fig. 4Comparison of weight gain between the two groups.
Data expressed in kilograms, and pooled using random-effects model. Significant weight gain found in atypical group (P= 0.04). Heterogeneity χ2= 29.79 (d.f. = 6)P<0.001,I 2= 79.9%. EPGN, Early Psychosis Global Network; GRNS, German Research Network on Schizophrenia; EUFEST, European First-Episode Schizophrenia Trial.
For an analysis of extrapyramidal side-effects, nine studies with 1341 participants were pooled. As described previously, most of the studies utilised the high-potency antipsychotic haloperidol and only two used zuclopenthixol and chlorpromazine. Rating scales reported varied, with five studies using the Simpson–Angus Scale (SAS), Reference Sanger, Lieberman, Tohen, Grundy, Beasley and Tollefson13,Reference Crespo-Facorro, Pérez-Iglesias, Ramirez-Bonilla, Martínez-García, Llorca and Luis Vázquez-Barquero28,Reference Gaebel, Riesbeck, Wölwer, Klimke, Eickhoff and von Wilmsdorff30,Reference Lieberman, Phillips, Gu, Stroup, Zhang and Kong36,Reference Green, Lieberman, Hamer, Glick, Gur and Kahn38 three studies the Extrapyramidal Symptom Rating Scale (ESRS), Reference Emsley24,Reference Schooler, Rabinowitz, Davidson, Emsley, Harvey and Kopala26,Reference Glenthøj, Glenthøj, Mackeprang, Pagsberg, Hemmingsen and Jernigan39 and one the St Hans Rating Scale for Extrapyramidal Syndrome (SHRS). Reference Kahn, Fleischhacker, Boter, Davidson, Vergouwe and Keet22 There are differences between the rating scales, but all of them include an objective evaluation of parkinsonism. The SHRS and ESRS both rate dystonia and akathisia, and the latter also includes a questionnaire to assess the subjective experience of extrapyramidal signs. In order to make these different scales comparable, only parkinsonism scores were extracted from these two scales when possible, and effect sizes were standardised. This was not possible in one study which reported global ESRS Reference Glenthøj, Glenthøj, Mackeprang, Pagsberg, Hemmingsen and Jernigan39 and in another one in which we used total ESRS at end-point (24 months) of observed cases provided by one of the authors. Reference Schooler, Rabinowitz, Davidson, Emsley, Harvey and Kopala26 We repeated this analysis excluding them. It should be noted that the results of this analysis apply to parkinsonism and not necessarily to other extrapyramidal symptoms such as akathisia. We found a significant advantage of atypicals over typicals as shown inFig. 5(s.d. = –0.38 favouring atypicals,P<0.001). Heterogeneity using chi-squared was not statistically significant (P= 0.14), and a fixed-effects analysis left the results substantially unchanged (s.d. = –0.37, 95% CI –0.48 to –0.25). Meta-regression analyses looking at dose of typical antipsychotics, amount of time exposed, and proportion of individuals receiving risperidone or amisulpride did not produce any statistically significant results. Exclusion of the two studies from which global ESRS scores were used did not substantially change the results.

Fig. 5Extrapyramidal side-effects in both groups using standardised mean differences.
A highly significant difference favouring atypicals was found (P<0.001). Note that all individual trials favour atypicals. Heterogeneity χ2= 12.3 (d.f. = 8)P= 0.14,I 2= 35%. EPGN, Early Psychosis Global Network; GRNS, German Research Network on Schizophrenia; EUFEST, European First-Episode Schizophrenia Trial.
Discussion
Main findings
We did not find a significant difference between atypical and typical antipsychotics in discontinuation rates. Discontinuation seems an appealing concept reflecting both efficacy of a drug and side-effects, but unfortunately there does not appear to be a consensus defining what exactly discontinuing a drug is: definitions of discontinuation ranged from irregularities in the adherence to a medication regime (e.g., taking lower doses of the assigned antipsychotic than prescribed for a period of 2 weeks as in Kahnet al) Reference Kahn, Fleischhacker, Boter, Davidson, Vergouwe and Keet22 to total discontinuation of the study drug (as in Schooleret al) Reference Schooler, Rabinowitz, Davidson, Emsley, Harvey and Kopala26 in the studies included in this meta-analysis. It is possible that a number of factors underpin these variations in what is defined as ‘discontinuation’ and this could be one of the causes of the significant statistical heterogeneity present in that comparison. Since only one study explicitly defined discontinuation in the context of being the primary outcome, Reference Kahn, Fleischhacker, Boter, Davidson, Vergouwe and Keet22 we were not able to analyse whether variation in the definition of discontinuation had an effect on the pooled outcome.
We found no significant difference between atypical and typical antipsychotics in acute symptomatic effect. Individuals with first-episode psychosis usually show a good symptomatic response to antipsychotic treatment, Reference Robinson, Woerner, Alvir, Geisler, Koreen and Sheitman11 and this may have introduced a ceiling effect that complicated comparisons. However, even if additional studies had been included such that the power of the analysis was increased, the estimated effect size of s.d. = 0.1 (equivalent to two points on the PANSS scale using the variance found in the biggest study included) Reference Schooler, Rabinowitz, Davidson, Emsley, Harvey and Kopala26 suggests that even if a significant difference could be identified it may not be clinically meaningful. A previously published study found that a one-step change in the Clinical Global Improvement scale (allegedly a more ‘clinically meaningful’ scale) required a 15-point change on the PANSS. Reference Leucht, Kane, Etschel, Kissling, Hamann and Engel40 A meta-analysis of treatment in chronic schizophrenia found an advantage for atypicals only when compared with high doses of typicals. Reference Geddes, Freemantle, Harrison and Bebbington1 In the present study, although the doses of typical antipsychotics prescribed in the studies we analysed were low, there was a similar trend for atypicals to be superior when the comparison involved higher doses of typicals. Although this observation was not significant, it supports the notion that when using typical antipsychotics in individuals with a first episode, lower doses of medication are indicated compared with individuals with chronic schizophrenia.
Although there were no significant differences between atypicals and typicals in discontinuation rates or symptom control, we did find differences in their side-effect profile. In line with previous studies Reference Alvarez-Jiménez, González-Blanch, Crespo-Facorro, Hetrick, Rodríguez-Sánchez and Pérez-Iglesias41,Reference Park, Ross-Degnan, Adams, Sabin, Kanavos and Soumerai42 our meta-analysis found that, on average, participants prescribed an atypical antipsychotic would gain 2 kg more than those on typicals, whereas those prescribed a typical antipsychotic would rate 0.4 standard deviations higher in the extrapyramidal scales (e.g. approximately one extra point in the SAS if using the data from one of the studies included). Reference Crespo-Facorro, Pérez-Iglesias, Ramirez-Bonilla, Martínez-García, Llorca and Luis Vázquez-Barquero28 We did not find a correlation between these findings and the use of specific atypical drugs that have been particularly associated with weight gain or extrapyramidal side-effects. This does not mean we can exclude any differences between the atypicals in their side-effect profiles in first-episode participants as this meta-analysis was not designed to look at within-class differences, and our meta-regression analysis was probably underpowered. As most of the studies we analysed included haloperidol, there is a possibility that the difference in extrapyramidal side-effects between atypicals and low-potency typical antipsychotics is less marked, as is the case in people with chronic schizophrenia. Reference Leucht, Wahlbeck, Hamann and Kissling2
Future research
This meta-analysis leaves many questions unanswered. First, it would have been interesting to look at differences in relapse rates between atypicals and typicals. We decided not to pool this outcome as there were too few studies reporting it. From the long-term studies included in this meta-analysis, three reported relapse rates in participants who achieved remission Reference Schooler, Rabinowitz, Davidson, Emsley, Harvey and Kopala26,Reference Gaebel, Riesbeck, Wölwer, Klimke, Eickhoff and von Wilmsdorff30,Reference Green, Lieberman, Hamer, Glick, Gur and Kahn38 and one reported readmissions to hospital in participants recruited from an in-patient unit. Reference Lieberman, Phillips, Gu, Stroup, Zhang and Kong36 Of these four, three reported no significant differences. Reference Gaebel, Riesbeck, Wölwer, Klimke, Eickhoff and von Wilmsdorff30,Reference Lieberman, Phillips, Gu, Stroup, Zhang and Kong36,Reference Green, Lieberman, Hamer, Glick, Gur and Kahn38 The other trial, Reference Schooler, Rabinowitz, Davidson, Emsley, Harvey and Kopala26 which was the largest and longest, reported significantly lower relapse rates with risperidone compared with haloperidol (42.1%v.54.7% respectively), even though no differences were found in remission and medication adherence. As relapse is an important determinant of long-term outcome, this should be a serious consideration in the choice of antipsychotic. Further large long-term studies would be needed to verify this study's findings. Second, some of the most important risks with antipsychotics such as diabetes (seen with some atypicals) or tardive dyskinesia (commonly seen with typicals) usually appear after years of exposure to these medications. This makes it exceedingly unlikely that this will ever be studied using randomised controlled trials. However, these serious long-term outcomes should also be considered when deciding upon maintenance medication. Changes in glucose and lipid blood levels with antipsychotic medication could be seen as an intermediate outcome in metabolic complications, but the small proportion of studies reporting this outcome from the included studies discouraged us to perform a meta-analysis.
Recent criticisms have been raised about the validity of atypical antipsychotics as a group. Reference Tyrer and Kendall43,Reference Margolis44 This is backed mostly by two recent meta-analyses by Leuchtet al,who reported differences within the atypical antipsychotics Reference Leucht, Komossa, Rummel-Kluge, Corves, Hunger and Schmid45 and also when compared with typical antipsychotics. Reference Leucht, Corves, Arbter, Engel, Li and Davis4 As already mentioned, our meta-analysis was not designed to look at differences within the atypical antipsychotics. Nevertheless, it should be noted that almost all the drugs included in this meta-analysis were those that were reported as more effective than their comparisons for chronic schizophrenia in the above mentioned studies (namely olanzapine, risperidone, clozapine and amisulpride).
Implications
This meta-analysis revealed no significant differences between typical and atypical antipsychotics in discontinuation rates or in short-term symptomatic response in individuals with first-episode psychosis. However, treatment with atypical antipsychotics was associated with relatively more weight gain, whereas treatment with typicals was associated with a greater incidence of extrapyramidal side-effects. Choice of antipsychotic drug in the treatment of first-episode psychosis may thus be more influenced by side-effect profile than efficacy.
Acknowledgements
We are grateful to Dr Han Boter, Dr Warrick Brewer, Dr Juan Bustillo, Professor Robin Emsley, Professor Wolfgang Gaebel, Dr Ragy Girgis, Dr Birte Glenthøj, Professor Rene Kahn, Mr Keith Karcher, Professor Jeffrey Lieberman, Professor Jonathan Rabinowitz and Dr Mathias Riesbeck who provided unpublished data to the authors and clarified all the doubts we had. We would also like to thank Dr Daniel Stahl for statistical advice.








eLetters
No eLetters have been published for this article.