Abstract
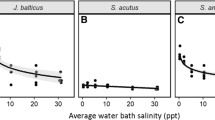
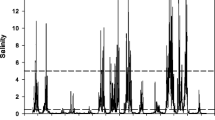
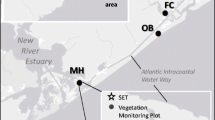
We examined patterns of habitat function (plant species richness), productivity (plant aboveground biomass and total C), and nutrient stocks (N and P in aboveground plant biomass and soil) in tidal marshes of the Satilla, Altamaha, and Ogeechee Estuaries in Georgia, USA. We worked at two sites within each salinity zone (fresh, brackish, and saline) in each estuary, sampling a transect from the creekbank to the marsh platform. In total, 110 plant species were found. Site-scale and plot-scale species richness decreased from fresh to saline sites. Standing crop biomass and total carbon stocks were greatest at brackish sites, followed by freshwater then saline sites. Nitrogen stocks in plants and soil decreased across sites as salinity increased, while phosphorus stocks did not differ between fresh and brackish sites but were lowest at salty sites. These results generally support past speculation about ecosystem change across the estuarine gradient, emphasizing that ecosystem function in tidal wetlands changes sharply across the relatively short horizontal distance of the estuary. Changes in plant distribution patterns driven by global changes such as sea level rise, changing climates, or fresh water withdrawal are likely to have strong impacts on a variety of wetland functions and services.








Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Baldwin, A.H. and I.A. Mendelssohn. 1998. Response of two oligohaline marsh communities to lethal and nonlethal disturbance.Oecologia116: 543–555. doi:10.1007/s004420050620.
Birch, J.B. and J.L. Cooley. 1982. Production and standing crop patterns of giant cutgrass (Zizaniopsis miliacea) in a fresh-water tidal marsh.Oecologia52: 230–235. doi:10.1007/BF00363842.
Costanza, R., R. dArge, R. deGroot, S. Farber, M. Grasso, B. Hannon, K. Limburg, S. Naeem, R.V. Oneill, J. Paruelo, R.G. Raskin, P. Sutton, and M. vandenBelt. 1997. The value of the world’s ecosystem services and natural capital.Nature387: 253–260. doi:10.1038/387253a0.
Costanza, R., O. Perez-Maqueo, M.L. Martinez, P. Sutton, S.J. Anderson, and K. Mulder. 2008. The value of coastal wetlands for hurricane protection.Ambio37: 241–248. doi:10.1579/0044-7447(2008)37[241:TVOCWF]2.0.CO;2.
Craft, C. 2007. Freshwater input structures soil properties, vertical accretion, and nutrient accumulation of Georgia and U.S. tidal marshes.Limnology and Oceanography52: 1220–1230.
Craft, C., J. Clough, J. Ehman, S. Joye, R. Park, S.C. Pennings, H. Guo, and M. Machmuller. 2009. Forecasting the effects of accelerated sea-level rise on tidal marsh ecosystem services.Frontiers in Ecology and the Environment7: 73–78. doi:10.1890/070219.
Crain, C.M. 2007. Shifting nutrient limitation and eutrophication effects in marsh vegetation across estuarine salinity gradients.Estuaries and Coasts30: 26–34.
Crain, C.M., B.R. Silliman, S.L. Bertness, and M.D. Bertness. 2004. Physical and biotic drivers of plant distribution across estuarine salinity gradients.Ecology85: 2539–2549. doi:10.1890/03-0745.
Dai, T. and R.G. Wiegert. 1996. Ramet population dynamics and net aerial primary productivity ofSpartina alterniflora.Ecology77: 276–288. doi:10.2307/2265677.
Dame, R.F. and P.D. Kenny. 1986. Variability ofSpartina alternifloraprimary production in the Euhaline North inlet estuary.Marine Ecology Progress Series32: 71–80. doi:10.3354/meps032071.
de Groot, R.S., M.A. Wilson, and R.M.J. Boumans. 2002. A typology for the classification, description and valuation of ecosystem functions, goods and services.Ecological Economics41: 393–408. doi:10.1016/S0921-8009(02)00089-7.
Eleuterius, L.N. and F.C. Lanning. 1987. Silica in relation to leaf decomposition ofJuncus roemerianus.Journal of Coastal Research3: 531–534.
Farber, S.C., R. Costanza, and M.A. Wilson. 2002. Economic and ecological concepts for valuing ecosystem services.Ecological Economics41: 375–392. doi:10.1016/S0921-8009(02)00088-5.
Frost, J.W., S. Tymeri, and C. Craft. 2009. Effects of nitrogen and phosphorus additions on primary production and invertebrate densities in a Georgia (USA) tidal freshwater marsh.Wetlands29: 196–203. doi:10.1672/07-79.1.
Gallagher, J.L., R.J. Reimold, R.A. Linthurst, and W.J. Pfeiffer. 1980. Aerial production, mortality, and mineral accumulation—export dynamics inSpartina alternifloraandJuncus roemerianusplant stands in a Georgia salt marsh.Ecology61: 303–312. doi:10.2307/1935189.
Greenberg, R., J.E. Maldonado, S. Droege, and M.V. McDonald. 2006. Tidal marshes: A global perspective on the evolution and conservation of their terrestrial vertebrates.BioScience56: 675–685. doi:10.1641/0006-3568(2006)56[675:TMAGPO]2.0.CO;2.
Hatton, R.S., R.D. Delaune, and W.H. Patrick. 1983. Sedimentation, accretion, and subsidence in marshes of Barataria Basin, Louisiana.Limnology and Oceanography28: 494–502.
Howard, R.J. and I.A. Mendelssohn. 1999. Salinity as a constraint on growth of oligohaline marsh macrophytes. II. Salt pulses and recovery potential.American Journal of Botany86: 795–806. doi:10.2307/2656701.
Howard, R.J. and I.A. Mendelssohn. 2000. Structure and composition of oligohaline marsh plant communities exposed to salinity pulses.Aquatic Botany68: 143–164. doi:10.1016/S0304-3770(00)00108-X.
Judd, F.W. and R.I. Lonard. 2002. Species richness and diversity of brackish and salt marshes in the Rio Grande Delta.Journal of Coastal Research18: 751–759.
Judd, F.W. and R.I. Lonard. 2004. Community ecology of freshwater, brackish and salt marshes of the Rio Grande delta.Texas Journal of Science56: 103–122.
King, G.M., M.J. Klug, R.G. Wiegert, and A.G. Chalmers. 1982. Relation of soil-water movement and sulfide concentration toSpartina alternifloraproduction in a Georgia Salt-Marsh.Science218: 61–63. doi:10.1126/science.218.4567.61.
Koerselman, W. and A.F.M. Meuleman. 1996. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation.Journal of Applied Ecology33: 1441–1450. doi:10.2307/2404783.
Kunza, A.E. and S.C. Pennings. 2008. Patterns of plant diversity in Georgia and Texas salt marshes.Estuaries and Coasts31: 673–681.
Maricle, B.R., R.W. Lee, C.E. Hellquist, O. Kiirats, and G.E. Edwards. 2007. Effects of salinity on chlorophyll fluorescence and CO2 fixation in C-4 estuarine grasses.Photosynthetica45: 433–440. doi:10.1007/s11099-007-0072-7.
Morse, J.L., J.P. Megonigal, and M.R. Walbridge. 2004. Sediment nutrient accumulation and nutrient availability in two tidal freshwater marshes along the Mattaponi River, Virginia, USA.Biogeochemistry69: 175–206. doi:10.1023/B:BIOG.0000031077.28527.a2.
Naidoo, G. and J. Kift. 2006. Responses of the saltmarsh rushJuncus kraussiito salinity and waterlogging.Aquatic Botany84: 217–225. doi:10.1016/j.aquabot.2005.10.002.
Odum, W.E., T.J. Smith, J.K. Hoover, and C.C. McIvor. 1984. The ecology of tidal freshwater marshes of the United States East Coast: A community profile. Washington, DC:U.S. Fish and Wildlife Service Report FWS/OBS-83/17.
Odum, W.E. 1998. Comparative ecology of tidal freshwater and salt marshes.Annual Review of Ecology and Systematics19: 147–176.
Perry, J.E. and R.B. Atkinson. 1997. Plant diversity along a salinity gradient of four marshes on the York and Pamunkey Rivers in Virginia.Castanea62: 112–118.
Schubauer, J.P. and C.S. Hopkinson. 1984. Above- and belowground emergent macrophyte production and turnover in a coastal marsh ecosystem, Georgia.Limnology and Oceanography29: 1052–1065.
Sommers, L.E. and D.W. Nelson. 1972. Determination of total phosphorus in soils: A rapid perchloric acid digestion procedure.Soil Science Society of America Proceedings36: 902–904.
Udell, H.F., J. Zarudsky, T.E. Doheny, and P.R. Burkhol. 1969. Productivity and nutrient values of plants growing in salt marshes of the town of Hempstead, Long Island.Bulletin of the Torrey Botanical Club96: 42–51. doi:10.2307/2484006.
Vitousek, P.M. and R.W. Howarth. 1991. Nitrogen limitation on land and in the Sea—How can it occur?Biogeochemistry13: 87–115. doi:10.1007/BF00002772.
Weston, N.B., R.E. Dixon, and S.B. Joye. 2006. Ramifications of increased salinity in tidal freshwater sediments: Geochemistry and microbial pathways of organic matter mineralization.Journal of Geophysical Research: Biogeosciences111: G01009.
White, D.A., J.M. Trapani, L.B. Thien, and T.E. Weiss. 1978. Productivity and decomposition of dominant salt-marsh plants in Louisiana.Ecology59: 751–759. doi:10.2307/1938779.
Acknowledgments
We thank Ken Helm, Daniel Saucedo, and Alana Lynes for help in the field, and the US EPA STAR program (RD 83222001-0 to C. Craft) and NSF (OCE99-82133, OCE06-20959) for funding. This is contribution number 990 of the University of Georgia Marine Institute. This work is a contribution of the Georgia Coastal Ecosystems LTER program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Appendix 1
Geographic coordinates of field sites (Logger = location of water column salinity measurements with permanently deployed loggers). (DOC 51.0 kb)
Appendix 2
Summary of two-factor analyses of variance for the effects of salinity zone and estuary on plant species richness. *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001. (DOC 41.0 kb)
Appendix 3
Summary of analyses of variance for the effects of salinity zone and estuary on soil C, N, and P. *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001. (DOC 42.0 kb)
Appendix 4
Summary of three-factor analyses of variance for the effects of salinity zone, estuary, and elevation on plant height, biomass, C, N, and P. *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001. (DOC 45.0 kb)
Rights and permissions
About this article
Cite this article
Więski, K., Guo, H., Craft, C.B.et al.Ecosystem Functions of Tidal Fresh, Brackish, and Salt Marshes on the Georgia Coast. Estuaries and Coasts33,161–169 (2010). https://doi.org/10.1007/s12237-009-9230-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI:https://doi.org/10.1007/s12237-009-9230-4