This article has multiple issues.Please helpimprove itor discuss these issues on thetalk page.(Learn how and when to remove these messages)
|
Immunotherapyorbiological therapyis the treatment ofdiseaseby activating or suppressing theimmune system.Immunotherapies designed to elicit or amplify an immune response are classified asactivation immunotherapies,while immunotherapies that reduce or suppress are classified assuppression immunotherapies.Immunotherapy is under preliminary research for its potential to treat various forms ofcancer.[1][2][3][4]
| Immunotherapy | |
|---|---|
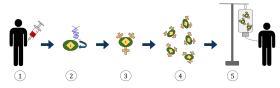 The diagram above represents the process of chimeric antigen receptor T-cell therapy (CAR), this is a method of immunotherapy, which is a growing practice in the treatment of cancer. The final result should be a production of equipped T-cells that can recognize and fight the infected cancer cells in the body.
| |
| MeSH | D007167 |
| OPS-301 code | 8-03 |
Cell-based immunotherapies are effective for some cancers.[5][6]Immune effector cells such aslymphocytes,macrophages,dendritic cells,natural killer cells,andcytotoxic T lymphocyteswork together to defend the body against cancer by targeting abnormal antigens expressed on the surface of tumor cells. Vaccine-induced immunity to COVID-19 relies mostly on an immunomodulatory T-cell response.[7]
Therapies such asgranulocyte colony-stimulating factor(G-CSF),interferons,imiquimodand cellular membrane fractions frombacteriaare licensed for medical use. Others includingIL-2,IL-7,IL-12,variouschemokines,synthetic cytosine phosphate-guanosine (CpG) oligodeoxynucleotides andglucansare involved in clinical and preclinical studies.
Immunomodulators
editImmunomodulators are the active agents of immunotherapy. They are a diverse array of recombinant, synthetic, and natural preparations.[8]
| Class | Example agents |
|---|---|
| Interleukins | IL-2,IL-7,IL-12 |
| Cytokines | Interferons,G-CSF |
| Chemokines | CCL3,CCL26,CXCL7 |
| Immunomodulatory imide drugs(IMiDs) | thalidomideand its analogues (lenalidomide,pomalidomide,andapremilast), BCG vaccine,[9][10]& Covid vaccines[11][12][7] |
| Other | cytosine phosphate-guanosine, oligodeoxynucleotides,glucans |
Activation immunotherapies
editCancer
editCancer treatment used to be focused on killing or removing cancer cells and tumours, with chemotherapy or surgery or radiation. In 2018 theNobel Prizein Physiology or Medicine was awarded toJames P. AllisonandTasuku Honjo"for their discovery of cancer therapy by inhibition of negative immune regulation." Cancer immunotherapy attempts to stimulate theimmune systemto destroy tumours. A variety of strategies are in use or are undergoing research and testing. Randomized controlled studies in different cancers resulting in significant increase in survival and disease free period have been reported[2]and its efficacy is enhanced by 20–30% when cell-based immunotherapy is combined with conventional treatment methods.[2]
One of the oldest forms of cancer immunotherapy is the use ofBCG vaccine,which was originally to vaccinate againsttuberculosisand later was found to be useful in the treatment ofbladder cancer.[13]BCG immunotherapy induces both local and systemic immune responses. The mechanisms by which BCG immunotherapy mediates tumor immunity have been widely studied, but they are still not completely understood.[14]
The use ofmonoclonal antibodiesin cancer therapy was first introduced in 1997 withrituximab,an anti-CD20 antibody for treatment of B cell lymphoma.[15]Since then several monoclonal antibodies have been approved for treatment of various haematological malignancies as well as for solid tumours.[16][17]
The extraction ofG-CSFlymphocytesfrom the blood and expanding in vitro against a tumour antigen before reinjecting the cells with appropriate stimulatorycytokines.The cells then destroy the tumour cells that express theantigen.[18]Topical immunotherapy utilizes an immune enhancement cream (imiquimod) which producesinterferon,causing the recipient's killerT cellsto destroywarts,[19]actinic keratoses,basal cell cancer,vaginal intraepithelial neoplasia,[20]squamous cell cancer,[21][22]cutaneous lymphoma,[23]and superficial malignant melanoma.[24]Injection immunotherapy ( "intralesional" or "intratumoural" ) uses mumps, candida, the HPV vaccine[25][26]ortrichophytinantigeninjections to treat warts (HPV induced tumours).
Adoptive cell transferhas been tested onlung[27]and other cancers, with greatest success achieved inmelanoma.
Dendritic cell-based pump-priming or vaccination
editDendritic cells (DC)can be stimulated to activate acytotoxicresponse towards anantigen.Dendritic cells, a type ofantigen-presenting cell,are harvested from the person needing the immunotherapy. These cells are then either pulsed with an antigen or tumour lysate ortransfectedwith aviral vector,causing them to display the antigen. Upon transfusion into the person, these activated cells present the antigen to the effector lymphocytes (CD4+ helper T cells,cytotoxicCD8+ T cellsandB cells). This initiates a cytotoxic response against tumour cells expressing the antigen (against which the adaptive response has now been primed). The first FDA-approved cell-based immunotherapy,[28]thecancer vaccineSipuleucel-Tis one example of this approach.[29]The Immune Response Corporation[30](IRC) developed this immunotherapy and licensed the technology to Dendreon, which obtained FDA clearance.
The current approaches forDC-based vaccinationare mainly based on antigen loading onin vitro-generated DCs frommonocytesorCD34+ cells, activating them with differentTLRligands,cytokinecombinations, and injecting them back to the patients. Thein vivotargeting approaches comprise administering specific cytokines (e.g.,Flt3L,GM-CSF) and targeting the DCs with antibodies to C-type lectin receptors or agonistic antibodies (e.g., anti-CD40) that are conjugated with antigen of interest. Multiple, next-generation anti-CD40 platforms are being actively developed.[31]Future approach may target DC subsets based on their specifically expressedC-type lectin receptorsorchemokine receptors.Another potential approach is the generation of genetically engineered DCs frominduced pluripotent stem cellsand use ofneoantigen-loaded DCs for inducing better clinical outcome.[32]
T-cell adoptive transfer
editAdoptive cell transferin vitrocultivates autologous, extracted T cells for later transfusion.[33]
Alternatively,Genetically engineered T cellsare created by harvesting T cells and then infecting the T cells with aretrovirusthat contains a copy of aT cell receptor(TCR) gene that is specialised to recognise tumour antigens. The virus integrates the receptor into the T cells'genome.The cells are expanded non-specifically and/or stimulated. The cells are then reinfused and produce an immune response against the tumour cells.[34]The technique has been tested on refractory stage IV metastatic melanomas[33]and advancedskin cancer.[35][36][37]The first FDA-approved CAR-T drug, Kymriah, used this approach. To obtain the clinical and commercial supply of this CAR-T, Novartis purchased the manufacturing plant, the distribution system and hired the production team that produced Sipuleucel-T developed by Dendreon and the Immune Response Corporation.[38]
Whether T cells are genetically engineered or not, before re-infusion, lympho-depletion of the recipient is required to eliminate regulatory T cells as well as unmodified, endogenous lymphocytes that compete with the transferred cells for homeostatic cytokines.[33][39][40][41]Lymphodepletion may be achieved bymyeloablativechemotherapy, to which total body irradiation may be added for greater effect.[42]Transferred cells multipliedin vivoand persisted in peripheral blood in many people, sometimes representing levels of 75% of all CD8+T cells at 6–12 months after infusion.[43]As of 2012[update],clinical trials for metastatic melanoma were ongoing at multiple sites.[44]Clinical responses to adoptive transfer of T cells were observed in patients with metastatic melanoma resistant to multiple immunotherapies.[45]
Checkpoint inhibitors
editAnti-PD-1/PD-L1and anti-CTLA-4 antibodies are the two types of checkpoint inhibitors currently available to patients. The approval of anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and anti-programmed cell death protein 1 (PD-1) antibodies for human use has already resulted in significant improvements in disease outcomes for various cancers.[46]
Although these molecules were originally discovered as molecules playing a role inT cell activationorapoptosis,subsequent preclinical research showed their important role in the maintenance of peripheral immune tolerance.[47]
Immune checkpoint inhibitors are approved to treat some patients with a variety of cancer types, includingmelanoma,breast cancer,bladder cancer,cervical cancer,colon cancer,lung cancerhead and neck cancer,orHodgkin lymphoma.[48][49]
These therapies have revolutionizedcancer immunotherapyas they showed for the first time in many years of research in metastaticmelanoma,which is considered one of the mostimmunogenichuman cancers, an improvement in overall survival, with an increasing group of patients benefiting long-term from these treatments, although caution remains needed for specific subgroups.[47][50][51]
The next generation of checkpoint inhibitors targets other receptors such as lymphocyte-activation gene 3 (LAG-3), T-cell immunoglobulin and mucin-domain containing-3 (TIM3), and T cell immunoreceptor with Ig and ITIM domains (TIGIT). Antibodies against these receptors have been evaluated in clinical studies, but have not yet been approved for widespread use.[52]
Immune enhancement therapy
editAutologous immune enhancement therapyuse a person's own peripheral blood-derivednatural killer cells,cytotoxic T lymphocytes, epithelial cells and other relevant immune cells are expandedin vitroand then re-infused.[53]The therapy has been tested againsthepatitis C,[54][55][56]chronic fatigue syndrome[57][58]andHHV6infection.[59]
Suppression immunotherapies
editImmune suppressiondampens an abnormalimmune responseinautoimmune diseasesor reduces a normalimmune responseto preventrejectionoftransplantedorgans or cells.
Immunosuppressive drugs
editImmunosuppressive drugs can be used to control the immune system with organ transplantation and with autoimmune disease. Immune responses depend on lymphocyte proliferation. Lymphocyte proliferation is the multiplication of lymphocyte cells used to fight and remember foreign invaders.[60]Cytostatic drugs are a type of immunosuppressive drug that aids in slowing down the growth of rapidly dividing cells. Another example of an immunosuppressive drug is Glucocorticoids which are more specific inhibitors of lymphocyte activation. Glucocorticoids work by emulating actions of natural actions of the body's adrenal glands to help suppress the immune system, which is helpful with autoimmune diseases|,[61]Alternatively, inhibitors of immunophilins more specifically target T lymphocyte activation, the process by which T-lymphocytes stimulate and begin to respond to a specific antigen,[62]There is also Immunosuppressive antibodies which target steps in the immune response to prevent the body from attacking its tissues, which is a problem with autoimmune diseases,[63]There are various other drugs that modulate immune responses and can be used to induce immune regulation. It was observed in a preclinical trial that regulation of the immune system by small immunosuppressive molecules such as vitamin D, dexamethasone, and curcumin could be helpful in preventing or treating chronic inflation. Given that the molecules are administered under a low-dose regimen and subcutaneously. A study provides a promising preclinical demonstration of the effectiveness and ease of preparation of Valrubicin-loaded immunoliposomes (Val-ILs) as a novel nanoparticle technology to target immunosuppressive cells. Val-ILs have the potential to be used as a precise and effective therapy based on targeted vesicle-mediated cell death of immunosuppressive cells.[64]
Immune tolerance
editThe body naturally does not launch an immune system attack on its own tissues. Models generally identifyCD4+ T-cellsat the centre of theautoimmune response.Loss of T-cell tolerance then unleashes B-cells and other immune effector cells on to the target tissue. The idealtolerogenic therapywould target the specific T-cell clones co-ordinating the autoimmune attack.[65]
Immune tolerancetherapies seek to reset the immune system so that the body stops mistakenly attacking its own organs or cells inautoimmune diseaseor accepts foreign tissue inorgan transplantation.[66]A recent[when?]therapeutic approach is the infusion ofregulatory immune cellsinto transplant recipients. The transfer of regulatory immune cells has the potential to inhibit the activity of effector.[67][68]
Creatingimmune tolerancereduces or eliminates the need for lifelong immunosuppression and attendant side effects. It has been tested on transplantations,rheumatoid arthritis,type 1 diabetesand other autoimmune disorders.
| Modality | Details | ||
|---|---|---|---|
| Non-antigen specific | Monoclonal Antibodies |
Depleting: |
Non-depleting: |
| Haematopoietic stem cell transplantation | Non-myeloablative | Myeloablative | |
| Mesenchymal stem cell transplantation | |||
| Regulatory T cell therapy | Non-antigen specific | Antigen-specific | |
| Low doseIL-2to expand regulatory T cells | |||
| Microbiome manipulation | |||
| Antigen specific | Peptide therapy | Subcutaneous, intradermal, transmucosal (oral, inhaled)
Tolerogenic dendritic cells, liposomes and nanoparticles | |
| Altered peptide ligands | |||
Allergen immunotherapy
editImmunotherapy can also be used to treatallergies.While allergy treatments (such asantihistaminesorcorticosteroids) treat allergic symptoms, immunotherapy can reduce sensitivity toallergens,lessening its severity. Allergen immunotherapy can also be referred to as allergen desensitization or hypo-sensitization.[71]Immunotherapy may produce long-term benefits.[72]Immunotherapy is partly effective in some people and ineffective in others, but it offers people with allergies a chance to reduce or stop their symptoms.[citation needed]
Subcutaneous allergen immunotherapy was first introduced in 1911 through the hypothesis that people with hay fever were sensitive to pollen from grass. A process was developed to create an extract by drawing out timothy pollen in distilled water and then boiling it. This was injected into patients in increasing doses to help alleviate symptoms.[73]
Allergen Immunotherapy is indicated for people who are extremely allergic or who cannot avoid specificallergensand when there is evidence of an IgE-mediated reaction that correlates with allergen symptoms. These IgE-mediated reactions can be identified via a blood IgE test or skin testing. If a specific IgE antibody is negative, there is no evidence that allergen immunotherapy will be effective for that patient.
However, there are risks associated with allergen immunotherapy as it is the administration of an agent the patient is known to be highly allergic to. Patients are at increased risk of fatalanaphylaxis,local reaction at the site of injection, or life-threatening systemic allergic reactions.[71]
A promising approach to treat food allergies is the use oforal immunotherapy(OIT). OIT consists in a gradual exposure to increasing amounts of allergen can lead to the majority of subjects tolerating doses of food sufficient to prevent reaction on accidental exposure.[74]Dosages increase over time, as the person becomes desensitized. This technique has been tested on infants to prevent peanut allergies.[75]
Helminthic therapies
editWhipwormova(Trichuris suis) andhookworm(Necator americanus) have been tested for immunological diseases and allergies, and have proved beneficial on multiple fronts, yet it is not entirely understood. Scientists have found that the immune response triggered by the burrowing of hookworm larvae to pass through the lungs and blood so the production of mast cells and specific antibodies are now present. They also reduce inflammation or responses ties to autoimmune diseases, but despite this, the hookworm's effects are considered to be negative typically.[76]Helminthic therapyhas been investigated as a treatment for relapsing remittingmultiple sclerosis,[77]Crohn's,[78][79][80]allergies and asthma.[81]While there is much to be learned about this, many researchers think that the change in the immune response is thanks to the parasites shifting to a more anti-inflammatory or regulatory system, which would in turn decrease inflammation and self inflicted immune damage as seen in Crohn's and multiple sclerosis. Specifically, MS patients saw lower relapse rates and calmer symptoms in some cases when experimenting with helminthic therapy.[82]Hypothesized mechanisms include re-polarisation of theTh1 / Th2response[83]and modulation of dendritic cell function.[84][85]The helminths downregulate the pro-inflammatory Th1 cytokines,interleukin-12(IL-12),interferon-gamma(IFN-γ) andtumor necrosis factor-alpha(TNF-α), while promoting the production of regulatory Th2 cytokines such asIL-10,IL-4,IL-5andIL-13.[83][86]
Co-evolution with helminths has shaped some of the genes associated withinterleukinexpression and immunological disorders, suchCrohn's,ulcerative colitisandceliac disease.Helminths' relationship to humans as hosts should be classified as mutualistic orsymbiotic.[87]In some ways, the relationship is symbiotic because the worms themselves need the host (humans) for survival, because this body supplies them with nutrients and a home. From another perspective, it could be reasoned that it is mutualistic, being that the above information about benefits in autoimmune disorders continues to remain true and supported. Also, some say that the worms can regulate gut bacteria.[88]Another possibility is one of this being a parasitic relationship, arguing that the possibile rosks of anemia and other disorders outweighs the benefits, yet this is significantly less supported, with the research alluding to the mutualitic and symbiotic approach being much more likely.
See also
editReferences
edit- ^"Immunotherapy | Memorial Sloan Kettering Cancer Center".mskcc.org.Archivedfrom the original on 2019-10-19.Retrieved2017-07-27.
- ^abcSyn NL, Teng MW, Mok TS, Soo RA (December 2017). "De-novo and acquired resistance to immune checkpoint targeting".The Lancet. Oncology.18(12): e731–e741.doi:10.1016/s1470-2045(17)30607-1.PMID29208439.
- ^Conforti L (February 2012). "The ion channel network in T lymphocytes, a target for immunotherapy".Clinical Immunology.142(2): 105–106.doi:10.1016/j.clim.2011.11.009.PMID22189042.
- ^Wang S, Zimmermann S, Parikh K, Mansfield AS, Adjei AA (August 2019)."Current Diagnosis and Management of Small-Cell Lung Cancer".Mayo Clinic Proceedings.94(8): 1599–1622.doi:10.1016/j.mayocp.2019.01.034.PMID31378235.
- ^Riley RS, June CH, Langer R, Mitchell MJ (March 2019)."Delivery technologies for cancer immunotherapy".Nature Reviews. Drug Discovery.18(3): 175–196.doi:10.1038/s41573-018-0006-z.PMC6410566.PMID30622344.
- ^Li Y, McBride DW, Tang Y, Doycheva D, Zhang JH, Tang Z (September 2023)."Immunotherapy as a treatment for Stroke: Utilizing regulatory T cells".Brain Hemorrhages.4(3): 147–153.doi:10.1016/j.hest.2023.02.003.ISSN2589-238X.
- ^abGeers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, et al. (May 2021)."SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees".Science Immunology.6(59): eabj1750.doi:10.1126/sciimmunol.abj1750.PMC9268159.PMID34035118.
- ^Rizk JG, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, Forthal DN (September 2020)."Pharmaco-Immunomodulatory Therapy in COVID-19".Drugs.80(13): 1267–1292.doi:10.1007/s40265-020-01367-z.PMC7372203.PMID32696108.
- ^"Immunomodulators and Their Side Effects".www.cancer.org.Archivedfrom the original on 2023-04-08.Retrieved2021-06-06.
- ^Martino A, Casetti R, Poccia F (January 2007). "Enhancement of BCG-induced Th1 immune response through Vgamma9Vdelta2 T cell activation with non-peptidic drugs".Vaccine.25(6): 1023–1029.doi:10.1016/j.vaccine.2006.09.070.PMID17118497.
- ^Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. (October 2020)."COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses ".Nature.586(7830): 594–599.Bibcode:2020Natur.586..594S.doi:10.1038/s41586-020-2814-7.PMID32998157.
- ^Woldemeskel BA, Garliss CC, Blankson JN (May 2021)."SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63".The Journal of Clinical Investigation.131(10).doi:10.1172/JCI149335.PMC8121504.PMID33822770.
- ^Fuge O,Vasdev N,Allchorne P, Green JS (2015)."Immunotherapy for bladder cancer".Research and Reports in Urology.7:65–79.doi:10.2147/RRU.S63447.PMC4427258.PMID26000263.
- ^Pettenati C, Ingersoll MA (October 2018). "Mechanisms of BCG immunotherapy and its outlook for bladder cancer".Nature Reviews. Urology.15(10): 615–625.doi:10.1038/s41585-018-0055-4.PMID29991725.S2CID49670901.
- ^Salles G, Barrett M, Foà R, Maurer J, O'Brien S, Valente N, et al. (October 2017)."Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience".Advances in Therapy.34(10): 2232–2273.doi:10.1007/s12325-017-0612-x.PMC5656728.PMID28983798.
- ^Hoos A (April 2016). "Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations".Nature Reviews. Drug Discovery.15(4): 235–247.doi:10.1038/nrd.2015.35.PMID26965203.S2CID54550859.
- ^Pento JT (November 2017)."Monoclonal Antibodies for the Treatment of Cancer".Anticancer Research.37(11): 5935–5939.doi:10.21873/anticanres.12040.PMC3288558.PMID29061772.
- ^Simpson RJ, Bigley AB, Agha N, Hanley PJ, Bollard CM (July 2017)."Mobilizing Immune Cells With Exercise for Cancer Immunotherapy".Exercise and Sport Sciences Reviews.45(3): 163–172.doi:10.1249/JES.0000000000000114.PMC6814300.PMID28418996.
- ^van Seters M, van Beurden M, ten Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, et al. (April 2008)."Treatment of vulvar intraepithelial neoplasia with topical imiquimod".The New England Journal of Medicine.358(14): 1465–1473.doi:10.1056/NEJMoa072685.PMID18385498.
- ^Buck HW, Guth KJ (October 2003). "Treatment of vaginal intraepithelial neoplasia (primarily low grade) with imiquimod 5% cream".Journal of Lower Genital Tract Disease.7(4): 290–293.doi:10.1097/00128360-200310000-00011.PMID17051086.S2CID44649376.
- ^Järvinen R, Kaasinen E, Sankila A, Rintala E (August 2009). "Long-term efficacy of maintenance bacillus Calmette-Guérin versus maintenance mitomycin C instillation therapy in frequently recurrent TaT1 tumours without carcinoma in situ: a subgroup analysis of the prospective, randomised FinnBladder I study with a 20-year follow-up".European Urology.56(2): 260–265.doi:10.1016/j.eururo.2009.04.009.PMID19395154.
- ^Davidson HC, Leibowitz MS, Lopez-Albaitero A, Ferris RL (September 2009)."Immunotherapy for head and neck cancer".Oral Oncology.45(9): 747–751.doi:10.1016/j.oraloncology.2009.02.009.PMC8978306.PMID19442565.
- ^Dani T, Knobler R (January 2009)."Extracorporeal photoimmunotherapy-photopheresis".Frontiers in Bioscience.14(14): 4769–4777.doi:10.2741/3566.PMID19273388.
- ^Eggermont AM, Schadendorf D (June 2009). "Melanoma and immunotherapy".Hematology/Oncology Clinics of North America.23(3): 547–64, ix–x.doi:10.1016/j.hoc.2009.03.009.PMID19464602.
- ^Chuang CM, Monie A, Wu A, Hung CF (May 2009)."Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects".Journal of Biomedical Science.16(1): 49.doi:10.1186/1423-0127-16-49.PMC2705346.PMID19473507.
- ^Pawlita M, Gissmann L (April 2009). "[Recurrent respiratory papillomatosis: indication for HPV vaccination?]".Deutsche Medizinische Wochenschrift(in German).134(Suppl 2): S100–S102.doi:10.1055/s-0029-1220219.PMID19353471.S2CID206295083.
- ^Kang N, Zhou J, Zhang T, Wang L, Lu F, Cui Y, et al. (August 2009). "Adoptive immunotherapy of lung cancer with immobilized anti-TCRgammadelta antibody-expanded human gammadelta T-cells in peripheral blood".Cancer Biology & Therapy.8(16): 1540–1549.doi:10.4161/cbt.8.16.8950.PMID19471115.S2CID23222462.
- ^Cheever MA, Higano CS (June 2011)."PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine".Clinical Cancer Research.17(11): 3520–3526.doi:10.1158/1078-0432.CCR-10-3126.PMID21471425.S2CID135120.
- ^Di Lorenzo G, Buonerba C, Kantoff PW (May 2011). "Immunotherapy for the treatment of prostate cancer".Nature Reviews. Clinical Oncology.8(9): 551–561.doi:10.1038/nrclinonc.2011.72.PMID21606971.S2CID5337484.
- ^"Sipuleucel-T: APC 8015, APC-8015, prostate cancer vaccine--Dendreon".Drugs in R&D.7(3): 197–201. 2006.doi:10.2165/00126839-200607030-00006.PMID16752945.S2CID6427074.
- ^Andersson H, Nyesiga B, Hermodsson T, Enell Smith K, Hägerbrand K, Lindstedt M, et al. (May 2024)."Next-generation CD40 agonists for cancer immunotherapy".Expert Opinion on Biological Therapy.24(5): 351–363.doi:10.1080/14712598.2024.2357714.PMID38764393.
- ^Sabado RL, Balan S, Bhardwaj N (January 2017)."Dendritic cell-based immunotherapy".Cell Research.27(1): 74–95.doi:10.1038/cr.2016.157.PMC5223236.PMID28025976.
- ^abcRosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME (April 2008)."Adoptive cell transfer: a clinical path to effective cancer immunotherapy".Nature Reviews. Cancer.8(4): 299–308.doi:10.1038/nrc2355.PMC2553205.PMID18354418.
- ^Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. (October 2006)."Cancer regression in patients after transfer of genetically engineered lymphocytes".Science.314(5796): 126–129.Bibcode:2006Sci...314..126M.doi:10.1126/science.1129003.PMC2267026.PMID16946036.
- ^Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. (June 2008)."Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1".The New England Journal of Medicine.358(25): 2698–2703.doi:10.1056/NEJMoa0800251.PMC3277288.PMID18565862.
- ^"2008 Symposium Program & Speakers".Cancer Research Institute. Archived fromthe originalon 2008-10-15.
- ^Highfield R (18 June 2008)."Cancer patient recovers after injection of immune cells".The Telegraph.Archived fromthe originalon 12 September 2008.Retrieved22 December2019.
- ^"Updated: Novartis buys Dendreon New Jersey plant".Fierce Pharma.20 December 2012.Archivedfrom the original on 2023-06-07.Retrieved2021-12-09.
- ^Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, et al. (March 2005)."CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells".Journal of Immunology.174(5): 2591–2601.doi:10.4049/jimmunol.174.5.2591.PMC1403291.PMID15728465.
- ^Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. (October 2005)."Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells".The Journal of Experimental Medicine.202(7): 907–912.doi:10.1084/jem.20050732.PMC1397916.PMID16203864.
- ^Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, et al. (July 2002)."T cell homeostatic proliferation elicits effective antitumor autoimmunity".The Journal of Clinical Investigation.110(2): 185–192.doi:10.1172/JCI15175.PMC151053.PMID12122110.
- ^Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. (November 2008)."Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens".Journal of Clinical Oncology.26(32): 5233–5239.doi:10.1200/JCO.2008.16.5449.PMC2652090.PMID18809613.
- ^Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. (October 2002)."Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes".Science.298(5594): 850–854.Bibcode:2002Sci...298..850D.doi:10.1126/science.1076514.PMC1764179.PMID12242449.
- ^Pilon-Thomas S, Kuhn L, Ellwanger S, Janssen W, Royster E, Marzban S, et al. (October 2012)."Efficacy of adoptive cell transfer of tumor-infiltrating lymphocytes after lymphopenia induction for metastatic melanoma".Journal of Immunotherapy.35(8): 615–620.doi:10.1097/CJI.0b013e31826e8f5f.PMC4467830.PMID22996367.
- ^Andersen R, Borch TH, Draghi A, Gokuldass A, Rana MA, Pedersen M, et al. (July 2018)."T cells isolated from patients with checkpoint inhibitor-resistant melanoma are functional and can mediate tumor regression".Annals of Oncology.29(7): 1575–1581.doi:10.1093/annonc/mdy139.PMID29688262.
- ^Seidel JA, Otsuka A, Kabashima K (2018-03-28)."Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations".Frontiers in Oncology.8:86.doi:10.3389/fonc.2018.00086.PMC5883082.PMID29644214.
- ^abHaanen JB, Robert C (2015). "Immune Checkpoint Inhibitors".Progress in Tumor Research.42:55–66.doi:10.1159/000437178.ISBN978-3-318-05589-4.PMID26382943.
- ^"Immune Checkpoint Inhibitors - National Cancer Institute".National Cancer Institute.2019-09-24.Archivedfrom the original on 2023-10-22.Retrieved2020-08-24.
- ^"Immunotherapy By Cancer Type".Cancer Research Institute.
- ^Queirolo P, Boutros A, Tanda E, Spagnolo F, Quaglino P (December 2019). "Immune-checkpoint inhibitors for the treatment of metastatic melanoma: a model of cancer immunotherapy".Seminars in Cancer Biology.59:290–297.doi:10.1016/j.semcancer.2019.08.001.hdl:2318/1717353.PMID31430555.
- ^Moyers JT, Glitza Oliva IC (2021). "Immunotherapy for Melanoma".Immunotherapy.Advances in Experimental Medicine and Biology. Vol. 1342. pp. 81–111.doi:10.1007/978-3-030-79308-1_3.ISBN978-3-030-79307-4.PMID34972963.
- ^Cai L, Li Y, Tan J, Xu L, Li Y (September 2023)."Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy".Journal of Hematology & Oncology.16(1): 101.doi:10.1186/s13045-023-01499-1.PMC10478462.PMID37670328.
- ^Manjunath SR, Ramanan G, Dedeepiya VD, Terunuma H, Deng X, Baskar S, et al. (January 2012)."Autologous immune enhancement therapy in recurrent ovarian cancer with metastases: a case report".Case Reports in Oncology.5(1): 114–118.doi:10.1159/000337319.PMC3364094.PMID22666198.
- ^Li Y, Zhang T, Ho C, Orange JS, Douglas SD, Ho WZ (December 2004)."Natural killer cells inhibit hepatitis C virus expression".Journal of Leukocyte Biology.76(6): 1171–1179.doi:10.1189/jlb.0604372.PMID15339939.
- ^Doskali M, Tanaka Y, Ohira M, Ishiyama K, Tashiro H, Chayama K, et al. (March 2011). "Possibility of adoptive immunotherapy with peripheral blood-derived CD3−CD56+ and CD3+CD56+ cells for inducing antihepatocellular carcinoma and antihepatitis C virus activity ".Journal of Immunotherapy.34(2): 129–138.doi:10.1097/CJI.0b013e3182048c4e.PMID21304407.S2CID26385818.
- ^Terunuma H, Deng X, Dewan Z, Fujimoto S, Yamamoto N (2008). "Potential role of NK cells in the induction of immune responses: implications for NK cell-based immunotherapy for cancers and viral infections".International Reviews of Immunology.27(3): 93–110.doi:10.1080/08830180801911743.PMID18437601.S2CID27557213.
- ^See DM, Tilles JG (1996). "alpha-Interferon treatment of patients with chronic fatigue syndrome".Immunological Investigations.25(1–2): 153–164.doi:10.3109/08820139609059298.PMID8675231.
- ^Ojo-Amaize EA, Conley EJ, Peter JB (January 1994). "Decreased natural killer cell activity is associated with severity of chronic fatigue immune dysfunction syndrome".Clinical Infectious Diseases.18(Suppl 1): S157–S159.doi:10.1093/clinids/18.Supplement_1.S157.PMID8148445.
- ^Kida K, Isozumi R, Ito M (December 2000). "Killing of human Herpes virus 6-infected cells by lymphocytes cultured with interleukin-2 or -12".Pediatrics International.42(6): 631–636.doi:10.1046/j.1442-200x.2000.01315.x.PMID11192519.S2CID11297558.
- ^"Lymphocyte Proliferation - an overview | ScienceDirect Topics".www.sciencedirect.com.Retrieved2024-05-10.
- ^"Lymphocyte Proliferation - an overview | ScienceDirect Topics".www.sciencedirect.com.Retrieved2024-05-10.
- ^Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2007-12-31).Molecular Biology of the Cell.doi:10.1201/9780203833445.ISBN978-0-203-83344-5.
- ^Qian Y, Dupps WJ, Meisler DM, Jeng BH (2010). "Epithelial Debridement for Epithelial Basement Membrane Abnormalities Associated with Endothelial Disorders".European Ophthalmic Review.04(1): 70.doi:10.17925/eor.2010.04.01.70.ISSN1756-1795.
- ^Georgievski A, Bellaye PS, Tournier B, Choubley H, Pais de Barros JP, Herbst M, et al. (May 2024)."Valrubicin-loaded immunoliposomes for specific vesicle-mediated cell death in the treatment of hematological cancers".Cell Death Dis.15(15(5):328): 328.doi:10.1038/s41419-024-06715-5.PMC11088660.PMID38734740.
- ^abRayner F, Isaacs JD (December 2018). "Therapeutic tolerance in autoimmune disease".Seminars in Arthritis and Rheumatism.48(3): 558–562.doi:10.1016/j.semarthrit.2018.09.008.PMID30348449.S2CID53034800.
- ^Rotrosen D, Matthews JB, Bluestone JA (July 2002)."The immune tolerance network: a new paradigm for developing tolerance-inducing therapies".The Journal of Allergy and Clinical Immunology.110(1): 17–23.doi:10.1067/mai.2002.124258.PMID12110811.S2CID30884739.
- ^Stolp J, Zaitsu M, Wood KJ (2019). "Immune Tolerance and Rejection in Organ Transplantation".Immunological Tolerance.Methods in Molecular Biology. Vol. 1899. pp. 159–180.doi:10.1007/978-1-4939-8938-6_12.ISBN978-1-4939-8936-2.PMID30649772.S2CID58542057.
- ^McMurchy AN, Bushell A, Levings MK, Wood KJ (August 2011)."Moving to tolerance: clinical application of T regulatory cells".Seminars in Immunology.Advances in Transplantation.23(4): 304–313.doi:10.1016/j.smim.2011.04.001.PMC3836227.PMID21620722.
- ^Baker KF, Isaacs JD (March 2014)."Prospects for therapeutic tolerance in humans".Current Opinion in Rheumatology.26(2): 219–227.doi:10.1097/BOR.0000000000000029.PMC4640179.PMID24378931.
- ^Cooles FA, Isaacs JD (August 2010). "Treating to re-establish tolerance in inflammatory arthritis - lessons from other diseases".Best Practice & Research. Clinical Rheumatology.Pharmacotherapy: Concepts of Pathogenesis and Emerging Treatments.24(4): 497–511.doi:10.1016/j.berh.2010.01.007.PMID20732648.
- ^abPersaud Y, Memon RJ, Savliwala MN (2024)."Allergy Immunotherapy".StatPearls.Treasure Island (FL): StatPearls Publishing.PMID30570988.Retrieved2024-05-10.
- ^Durham SR, Walker SM, Varga EM, Jacobson MR, O'Brien F, Noble W, et al. (August 1999)."Long-term clinical efficacy of grass-pollen immunotherapy".The New England Journal of Medicine.341(7): 468–475.doi:10.1056/NEJM199908123410702.PMID10441602.S2CID14629112.
- ^James C, Bernstein DI (February 2017)."Allergen immunotherapy: an updated review of safety".Current Opinion in Allergy & Clinical Immunology.17(1): 55–59.doi:10.1097/ACI.0000000000000335.ISSN1528-4050.PMC5644500.PMID27906697.
- ^MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, et al. (March 2017)."Omalizumab facilitates rapid oral desensitization for peanut allergy".The Journal of Allergy and Clinical Immunology.139(3): 873–881.e8.doi:10.1016/j.jaci.2016.08.010.PMC5369605.PMID27609658.S2CID3626708.
- ^"Oral immunotherapy for peanut allergy in young children".National Institutes of Health (NIH).2022-02-07.Archivedfrom the original on 2023-07-12.Retrieved2022-06-06.
- ^Loukas A, Prociv P (October 2001)."Immune responses in hookworm infections".Clinical Microbiology Reviews.14(4): 689–703, table of contents.doi:10.1128/CMR.14.4.689-703.2001.PMC89000.PMID11585781.
- ^Correale J, Farez M (February 2007). "Association between parasite infection and immune responses in multiple sclerosis".Annals of Neurology.61(2): 97–108.doi:10.1002/ana.21067.PMID17230481.S2CID1033417.
- ^Croese J, O'neil J, Masson J, Cooke S, Melrose W, Pritchard D, et al. (January 2006)."A proof of concept study establishing Necator americanus in Crohn's patients and reservoir donors".Gut.55(1): 136–137.doi:10.1136/gut.2005.079129.PMC1856386.PMID16344586.
- ^Reddy A, Fried B (January 2009). "An update on the use of helminths to treat Crohn's and other autoimmunune diseases".Parasitology Research.104(2): 217–221.doi:10.1007/s00436-008-1297-5.PMID19050918.S2CID19279688.
- ^Laclotte C, Oussalah A, Rey P, Bensenane M, Pluvinage N, Chevaux JB, et al. (December 2008). "[Helminths and inflammatory bowel diseases]".Gastroenterologie Clinique et Biologique(in French).32(12): 1064–1074.doi:10.1016/j.gcb.2008.04.030.PMID18619749.
- ^Zaccone P, Fehervari Z, Phillips JM, Dunne DW, Cooke A (October 2006)."Parasitic worms and inflammatory diseases".Parasite Immunology.28(10): 515–523.doi:10.1111/j.1365-3024.2006.00879.x.PMC1618732.PMID16965287.
- ^Donkers SJ, Kirkland MC, Charabati M, Osborne LC (2020)."Perspectives of People with Multiple Sclerosis About Helminth Immunotherapy".International Journal of MS Care.22(1): 43–51.doi:10.7224/1537-2073.2019-044.PMC7041615.PMID32123528.
- ^abBrooker S, Bethony J, Hotez PJ (2004)."Human hookworm infection in the 21st century".Advances in Parasitology.58:197–288.doi:10.1016/S0065-308X(04)58004-1.ISBN9780120317585.PMC2268732.PMID15603764.
- ^Fujiwara RT, Cançado GG, Freitas PA, Santiago HC, Massara CL, Dos Santos Carvalho O, et al. (2009)."Necator americanus infection: a possible cause of altered dendritic cell differentiation and eosinophil profile in chronically infected individuals".PLOS Neglected Tropical Diseases.3(3): e399.doi:10.1371/journal.pntd.0000399.PMC2654967.PMID19308259.
- ^Carvalho L, Sun J, Kane C, Marshall F, Krawczyk C, Pearce EJ (January 2009)."Review series on helminths, immune modulation and the hygiene hypothesis: mechanisms underlying helminth modulation of dendritic cell function".Immunology.126(1): 28–34.doi:10.1111/j.1365-2567.2008.03008.x.PMC2632707.PMID19120496.
- ^Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Riva S, Clerici M, et al. (June 2009)."Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions".The Journal of Experimental Medicine.206(6): 1395–1408.doi:10.1084/jem.20082779.PMC2715056.PMID19468064.
- ^Reynolds LA, Finlay BB, Maizels RM (November 2015)."Cohabitation in the Intestine: Interactions among Helminth Parasites, Bacterial Microbiota, and Host Immunity".Journal of Immunology.195(9): 4059–4066.doi:10.4049/jimmunol.1501432.PMC4617609.PMID26477048.
- ^Loke P, Lim YA (June 2015)."Helminths and the microbiota: parts of the hygiene hypothesis".Parasite Immunology.37(6): 314–23.doi:10.1111/pim.12193.PMC4428757.PMID25869420.
- ^Hong CH, Tang MR, Hsu SH, Yang CH, Tseng CS, Ko YC, et al. (September 2019). "Enhanced early immune response of leptospiral outer membrane protein LipL32 stimulated by narrow band mid-infrared exposure".Journal of Photochemistry and Photobiology. B, Biology.198:111560.Bibcode:2019JPPB..19811560H.doi:10.1016/j.jphotobiol.2019.111560.PMID31336216.S2CID198191485.
- ^Chang HY, Li MH, Huang TC, Hsu CL, Tsai SR, Lee SC, et al. (February 2015). "Quantitative proteomics reveals middle infrared radiation-interfered networks in breast cancer cells".Journal of Proteome Research.14(2): 1250–1262.doi:10.1021/pr5011873.PMID25556991.
- ^Nagaya T, Okuyama S, Ogata F, Maruoka Y, Choyke PL, Kobayashi H (May 2019)."Near infrared photoimmunotherapy using a fiber optic diffuser for treating peritoneal gastric cancer dissemination".Gastric Cancer.22(3): 463–472.doi:10.1007/s10120-018-0871-5.PMC7400986.PMID30171392.
- ^Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H (November 2011)."Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules".Nature Medicine.17(12): 1685–1691.doi:10.1038/nm.2554.PMC3233641.PMID22057348.
- ^Sato K, Sato N, Xu B, Nakamura Y, Nagaya T, Choyke PL, et al. (August 2016)."Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy".Science Translational Medicine.8(352): 352ra110.doi:10.1126/scitranslmed.aaf6843.PMC7780242.PMID27535621.
- ^Nagaya T, Nakamura Y, Sato K, Harada T, Choyke PL, Kobayashi H (June 2016)."Improved micro-distribution of antibody-photon absorber conjugates after initial near infrared photoimmunotherapy (NIR-PIT)".Journal of Controlled Release.232:1–8.doi:10.1016/j.jconrel.2016.04.003.PMC4893891.PMID27059723.
- ^Zhen Z, Tang W, Wang M, Zhou S, Wang H, Wu Z, et al. (February 2017). "Protein Nanocage Mediated Fibroblast-Activation Protein Targeted Photoimmunotherapy To Enhance Cytotoxic T Cell Infiltration and Tumor Control".Nano Letters.17(2): 862–869.Bibcode:2017NanoL..17..862Z.doi:10.1021/acs.nanolett.6b04150.PMID28027646.
