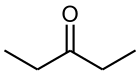
3-Pentanone(also known asdiethyl ketone) is a simple, symmetrical dialkyl ketone. It is a colorless liquidketonewith an odor like that ofacetone.It is soluble in about 25 parts water, but miscible with organic solvents.
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pentan-3-one | |
| Other names
Diethyl ketone, diethylketone, 3-pentanone, dimethyl acetone, propione, DEK, metacetone, methacetone, ethyl ketone fraction
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.265 |
| EC Number |
|
PubChemCID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1156 |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C5H10O | |
| Molar mass | 86.134g·mol−1 |
| Appearance | Colorless liquid[1] |
| Odor | Acetone-like[2] |
| Density | 0.81 g/cm3at 20 °C[2] |
| Melting point | −39 °C (−38 °F; 234 K)[2] |
| Boiling point | 102 °C (216 °F; 375 K)[2] |
| 35 g/L[2] | |
| Vapor pressure | 35 mmHg[1] |
| -58.14·10−6cm3/mol | |
| Hazards | |
| GHSlabelling: | |
 
| |
| Danger | |
| H225,H335,H336 | |
| P210,P233,P240,P241,P242,P243,P261,P271,P280,P303+P361+P353,P304+P340,P312,P370+P378,P403+P233,P403+P235,P405,P501 | |
| Flash point | 12.78 °C (55.00 °F; 285.93 K) |
| 425 °C (797 °F; 698 K) | |
| Explosive limits | 1.6%-6.4%[1] |
| NIOSH(US health exposure limits): | |
PEL(Permissible)
|
none[1] |
REL(Recommended)
|
TWA 200 ppm (705 mg/m3)[1] |
IDLH(Immediate danger)
|
N.D.[1] |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Uses
edit3-Pentanone is primarily used as starting material in chemical synthesis. A major application is in the industrial synthesis ofvitamin E.[3][4]It has also been used in thesynthesis of Oseltamivir(Tamiflu).
3-Pentanone itself finds some use as a specialty solvent in paint, although it is less common thanbutanone.
Syntheses
editKetonic decarboxylation route
edit3-Pentanone is produced byketonic decarboxylationofpropanoic acidusing metal oxide catalysts:
- 2 CH3CH2CO2H → (CH3CH2)2CO + CO2+ H2O
in the laboratory, the reaction can be conducted in atube furnace.[5]
Carbonylation route
editIt can also be prepared by combiningethylene,CO, and H2.[4]When the reaction is catalyzed bydicobalt octacarbonyl,water can be used as a source of hydrogen. A proposed intermediate is the ethylene-propionyl species [CH3C(O)Co(CO)3(ethylene)] which undergoes amigratory insertionto form [CH3COCH2CH2Co(CO)3]. The required hydrogen arises from thewater shift reaction.For details, see[6] If the water shift reaction is not operative, the reaction affords a polymer containing alternating carbon monoxide and ethylene units. Such aliphaticpolyketonesare more conventionally prepared usingpalladiumcatalysts.[7]
Safety
editTheTLVvalue for 3-pentanone is 200 ppm (705 mg/m3).[4]3-pentanone can be hazardous if it comes in contact with the skin or eyes, and can cause irritation of the skin and redness, watering, and itching of the eyes. This chemical can also cause nervous system or organ damage if ingested. Although considered stable, 3-pentanone is extremely flammable if exposed to flame, sparks, or another source of heat. For safety, it should be stored in a flammable materials cabinet away from heat or sources of ignition, preferably in a cool, well-ventilated area.[8]
See also
editReferences
edit- ^abcdefNIOSH Pocket Guide to Chemical Hazards."#0212".National Institute for Occupational Safety and Health(NIOSH).
- ^abcdeRecordin theGESTIS Substance Databaseof theInstitute for Occupational Safety and Health
- ^Müller, Marc-André; Schäfer, Christian; Litta, Gilberto; Klünter, Anna-Maria; Traber, Maret G.; Wyss, Adrian; Ralla, Theo; Eggersdorfer, Manfred; Bonrath, Werner (6 December 2022)."100 Years of Vitamin E: From Discovery to Commercialization"(PDF).European Journal of Organic Chemistry.2022(45).doi:10.1002/ejoc.202201190.
- ^abcHardo Siegel, Manfred Eggersdorfer "Ketones" inUllmann's Encyclopedia of Industrial Chemistry,Wiley-VCH, 2002 by Wiley-VCH, Wienheim.doi:10.1002/14356007.a15_077
- ^Furniss, Brian; Hannaford, Antony; Smith, Peter & Tatchell, Austin (1996).Vogel's Textbook of Practical Organic Chemistry(5th ed.). London: Longman Science & Technical. p.613.ISBN9780582462366.
- ^ Murata K.; Matsuda A. (1981)."Application of Homogeneous Water-Gas Shift Reaction III Further Study of the Hydrocarbonylation – A highly Selective Formation of Diethyl Keton from Ethene, CO and H2O ".Bulletin of the Chemical Society of Japan.54(7): 2089–2092.doi:10.1246/bcsj.54.2089.
- ^J. Liu; B.T. Heaton; J.A. Iggo & R. Whyman (2004). "The Complete Delineation of the Initiation, Propagation, and Termination Steps of the Carbomethoxy Cycle for the Carboalkoxylation of Ethene by Pd–Diphosphane Catalysts".Angew. Chem. Int. Ed.43(1): 90–94.doi:10.1002/anie.200352369.PMID14694480.
- ^Chemicals & Laboratory Equipment, Material Safety Data Sheet for 3-pentanoneArchived2010-01-02 at theWayback Machine,ScienceLab.com, updated 11/06/2008