Adipocytes,also known aslipocytesandfat cells,are thecellsthat primarily composeadipose tissue,specialized in storing energy asfat.[1]Adipocytes are derived frommesenchymal stem cellswhich give rise to adipocytes throughadipogenesis.Incell culture,adipocyte progenitors can also formosteoblasts,myocytesand other cell types.
| Adipocyte | |
|---|---|
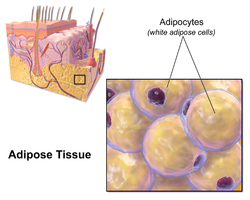 Illustration depicting white fat cells. | |
 Morphology of three different classes of adipocytes | |
| Details | |
| Identifiers | |
| Latin | adipocytus |
| MeSH | D017667 |
| TH | H2.00.03.0.01005 |
| FMA | 63880 |
| Anatomical terms of microanatomy | |
There are two types of adipose tissue,white adipose tissue(WAT) andbrown adipose tissue(BAT), which are also known as white and brown fat, respectively, and comprise two types of fat cells.
Structure
editWhite fat cells
editWhite fat cells contain a single largelipid dropletsurrounded by a layer of cytoplasm,and are known as unilocular. Thenucleusis flattened and pushed to the periphery. A typical fat cell is 0.1 mm in diameter[2]with some being twice that size, and others half that size. However, these numerical estimates of fat cell size depend largely on the measurement method and the location of the adipose tissue.[2]The fat stored is in a semi-liquid state, and is composed primarily oftriglycerides,andcholesteryl ester.White fat cells secrete many proteins acting asadipokinessuch asresistin,adiponectin,leptinandapelin.An average human adult has 30 billion fat cells with a weight of 30 lbs or 13.5 kg. If a child or adolescent gains sufficient excess weight, fat cells may increase in absolute number until age twenty-four.[3]If an adult (who never was obese as a child or adolescent) gains excess weight, fat cells generally increase in size, not number, though there is some inconclusive evidence suggesting that the number of fat cells might also increase if the existing fat cells become large enough (as in particularly severe levels of obesity).[3]The number of fat cells is difficult to decrease through dietary intervention, though some evidence suggests that the number of fat cells can decrease if weight loss is maintained for a sufficiently long period of time (>1 year; though it is extremely difficult for people with larger and more numerous fat cells to maintain weight loss for that long a time).[3]
A large meta-analysis has shown that white adipose tissue cell size is dependent on measurement methods, adipose tissue depots, age, and body mass index; for the same degree of obesity, increases in fat cell size were also associated with the dysregulations in glucose and lipid metabolism.[2]
Brown fat cells
editBrown fat cellsarepolyhedralin shape. Brown fat is derived from dermatomyocyte cells. Unlikewhite fatcells, these cells have considerable cytoplasm, with severallipiddroplets scattered throughout, and are known as multilocular cells. The nucleus is round and, although eccentrically located, it is not in the periphery of the cell. The brown color comes from the large quantity ofmitochondria.Brown fat, also known as "baby fat," is used to generate heat.
Marrow fat cells
editMarrow adipocytes are unilocular like white fat cells. Themarrow adipose tissuedepot is poorly understood in terms of its physiologic function and relevance to bone health. Marrow adipose tissue expands in states of low bone density but additionally expands in the setting of obesity.[4]Marrow adipose tissue response to exercise approximates that ofwhite adipose tissue.[4][5][6][7]Exercise reduces both adipocyte size as well as marrow adipose tissue volume, as quantified byMRIorμCT imagingof bone stained with the lipid binderosmium.
Development
editPre-adipocytesare undifferentiatedfibroblaststhat can be stimulated to form adipocytes. Studies have shed light into potential molecular mechanisms in the fate determination of pre-adipocytes although the exact lineage of adipocyte is still unclear.[8][9]The variation of body fat distribution resulting from normal growth is influenced by nutritional and hormonal status dependent on intrinsic differences in cells found in each adipose depot.[10]
Mesenchymal stem cellscan differentiate into adipocytes,connective tissue,muscle orbone.[1]
The precursor of the adult cell is termed alipoblast,and a tumor of this cell type is known as alipoblastoma.[11]
Function
editCell turnover
editFat cells in some mice have been shown to drop in count due to fasting and other properties were observed when exposed to cold.[12]
If the adipocytes in the body reach their maximum capacity of fat, they may replicate to allow additional fat storage.
Adult rats of various strains became obese when they were fed a highly palatable diet for several months. Analysis of their adipose tissue morphology revealed increases in both adipocyte size and number in most depots. Reintroduction of an ordinarychow diet[13]to such animals precipitated a period of weight loss during which only mean adipocyte size returned to normal. Adipocyte number remained at the elevated level achieved during the period of weight gain.[14]
According to some reports and textbooks, the number of adipocytes can increase in childhood and adolescence, though the amount is usually constant in adults. Individuals who become obese as adults, rather than as adolescents, have no more adipocytes than they had before.[15]
People who have been fat since childhood generally have an inflated number of fat cells. People who become fat as adults may have no more fat cells than their lean peers, but their fat cells are larger. In general, people with an excess of fat cells find it harder to lose weight and keep it off than the obese who simply have enlarged fat cells.[3]
Body fat cells have regional responses to the overfeeding that was studied in adult subjects. In the upper body, an increase of adipocyte size correlated with upper-body fat gain; however, the number of fat cells was not significantly changed. In contrast to the upper body fat cell response, the number of lower-body adipocytes did significantly increase during the course of experiment. Notably, there was no change in the size of the lower-body adipocytes.[16]
Approximately 10% of fat cells are renewed annually at all adult ages and levels of body mass index without a significant increase in the overall number of adipocytes in adulthood.[15]
Adaptation
editObesityis characterized by the expansion of fat mass, through adipocyte size increase (hypertrophy) and, to a lesser extent, cell proliferation (hyperplasia).[17][2]In thefatty tissueof obese individuals, there is increased production of metabolism modulators, such asglycerol,hormones,macrophage-stimulatingchemokines,andpro-inflammatory cytokines,leading to the development ofinsulin resistance.[18]Production of these modulators and the resultingpathogenesisof insulin resistance are probably caused by adipocytes as well asimmune systemmacrophages that infiltrate the tissue.[19]
Fat production in adipocytes is strongly stimulated byinsulin.By controlling the activity of thepyruvate dehydrogenaseand theacetyl-CoA carboxylaseenzymes, insulin promotes unsaturatedfatty acid synthesis.It also promotesglucose uptakeand inducesSREBF1,which activates the transcription of genes that stimulatelipogenesis.[20]
SREBF1(sterolregulatory element-bindingtranscription factor1) is a transcription factor synthesized as an inactiveprecursor proteininserted into theendoplasmic reticulum (ER)membrane by two membrane-spanninghelices.Also anchored in the ER membrane isSCAP(SREBF-cleavage activating protein), which binds SREBF1. The SREBF1-SCAP complex is retained in the ER membrane byINSIG1(insulin-induced gene 1 protein). When sterol levels are depleted, INSIG1 releases SCAP and the SREBF1-SCAP complex can be sorted into transportvesiclescoated by thecoatomerCOPIIthat are exported to theGolgi apparatus.In the Golgi apparatus, SREBF1 is cleaved and released as a transcriptionally active mature protein. It is then free totranslocateto thenucleusand activate the expression of its target genes.[21]
Clinical studies have repeatedly shown that even though insulin resistance is usually associated with obesity, the membranephospholipidsof the adipocytes of obese patients generally still show an increased degree of fatty acid unsaturation.[22]This seems to point to an adaptive mechanism that allows the adipocyte to maintain its functionality, despite the increased storage demands associated with obesity and insulin resistance.
A study conducted in 2013[22]found that, while INSIG1 and SREBF1mRNAexpression was decreased in the adipose tissue of obese mice and humans, the amount of active SREBF1 was increased in comparison with normal mice and non-obese patients. This downregulation of INSIG1 expression combined with the increase of mature SREBF1 was also correlated with the maintenance of SREBF1-target gene expression. Hence, it appears that, by downregulating INSIG1, there is a resetting of the INSIG1/SREBF1 loop, allowing for the maintenance of active SREBF1 levels. This seems to help compensate for the anti-lipogenic effects of insulin resistance and thus preserve adipocyte fat storage abilities and availability of appropriate levels of fatty acid unsaturation in face of the nutritional pressures of obesity.
Endocrine role
editAdipocytes can synthesizeestrogensfromandrogens,[23]potentially being the reason why beingunderweightoroverweightare risk factors forinfertility.[24]Additionally, adipocytes are responsible for the production of the hormoneleptin.Leptin is important in regulation of appetite and acts as a satiety factor.[25]
See also
editReferences
edit- ^abBirbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (August 2013)."Role of pericytes in skeletal muscle regeneration and fat accumulation".Stem Cells and Development.22(16): 2298–2314.doi:10.1089/scd.2012.0647.PMC3730538.PMID23517218.
- ^abcdYe RZ, Richard G, Gévry N, Tchernof A, Carpentier AC (January 2022)."Fat Cell Size: Measurement Methods, Pathophysiological Origins, and Relationships With Metabolic Dysregulations".Endocrine Reviews.43(1): 35–60.doi:10.1210/endrev/bnab018.PMC8755996.PMID34100954.
- ^abcdPool R (2001).Fat: fighting the obesity epidemic.Oxford [Oxfordshire]: Oxford University Press. pp.68.ISBN978-0-19-511853-7.
- ^abStyner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, et al. (August 2017)."Exercise Decreases Marrow Adipose Tissue Through ß-Oxidation in Obese Running Mice".Journal of Bone and Mineral Research.32(8): 1692–1702.doi:10.1002/jbmr.3159.PMC5550355.PMID28436105.
- ^Pagnotti GM, Styner M (2016)."Exercise Regulation of Marrow Adipose Tissue".Frontiers in Endocrinology.7:94.doi:10.3389/fendo.2016.00094.PMC4943947.PMID27471493.
- ^Styner M, Pagnotti GM, Galior K, Wu X, Thompson WR, Uzer G, et al. (August 2015)."Exercise Regulation of Marrow Fat in the Setting of PPARγ Agonist Treatment in Female C57BL/6 Mice".Endocrinology.156(8): 2753–2761.doi:10.1210/en.2015-1213.PMC4511140.PMID26052898.
- ^Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, et al. (July 2014)."Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise".Bone.64:39–46.doi:10.1016/j.bone.2014.03.044.PMC4041821.PMID24709686.
- ^Coskun H, Summerfield TL, Kniss DA, Friedman A (July 2010). "Mathematical modeling of preadipocyte fate determination".Journal of Theoretical Biology.265(1): 87–94.Bibcode:2010JThBi.265...87C.doi:10.1016/j.jtbi.2010.03.047.PMID20385145.
- Lay summary in:"Scientists closer to finding what causes the birth of a fat cell".ScienceDaily.August 18, 2010.
- ^Coskun H, Summerfield TL, Kniss DA, Friedman A (July 2010). "Mathematical modeling of preadipocyte fate determination".Journal of Theoretical Biology.265(1): 87–94.Bibcode:2010JThBi.265...87C.doi:10.1016/j.jtbi.2010.03.047.PMID20385145.
- ^Fried SK, Lee MJ, Karastergiou K (July 2015)."Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology".Obesity(Review).23(7): 1345–1352.doi:10.1002/oby.21133.PMC4687449.PMID26054752.
- ^Hong R, Choi DY, Do NY, Lim SC (July 2008). "Fine-needle aspiration cytology of a lipoblastoma: a case report".Diagnostic Cytopathology.36(7): 508–511.doi:10.1002/dc.20826.PMID18528880.S2CID22668394.
- ^Ding H, Zheng S, Garcia-Ruiz D, Hou D, Wei Z, Liao Z, et al. (May 2016)."Fasting induces a subcutaneous-to-visceral fat switch mediated by microRNA-149-3p and suppression of PRDM16".Nature Communications.7:11533.Bibcode:2016NatCo...711533D.doi:10.1038/ncomms11533.PMC4895052.PMID27240637.
- ^Warden CH, Fisler JS (April 2008)."Comparisons of diets used in animal models of high-fat feeding".Cell Metabolism.7(4): 277.doi:10.1016/j.cmet.2008.03.014.PMC2394560.PMID18396128.
Regular chow is composed of agricultural byproducts, such as ground wheat, corn, or oats, alfalfa and soybean meals, a protein source such as fish, and vegetable oil and is supplemented with minerals and vitamins. Thus, chow is a high fiber diet containing complex carbohydrates, with fats from a variety of vegetable sources. Chow is inexpensive to manufacture and is palatable to rodents.
- ^Faust IM, Johnson PR, Stern JS, Hirsch J (September 1978). "Diet-induced adipocyte number increase in adult rats: a new model of obesity".The American Journal of Physiology.235(3): E279–E286.doi:10.1152/ajpendo.1978.235.3.E279.PMID696822.S2CID7744250.
- ^abSpalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. (June 2008). "Dynamics of fat cell turnover in humans".Nature.453(7196): 783–787.Bibcode:2008Natur.453..783S.doi:10.1038/nature06902.PMID18454136.S2CID4431237.
- ^Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD (October 2010)."Regional differences in cellular mechanisms of adipose tissue gain with overfeeding".Proceedings of the National Academy of Sciences of the United States of America.107(42): 18226–18231.doi:10.1073/pnas.1005259107.PMC2964201.PMID20921416.
- ^Blüher M (June 2009). "Adipose tissue dysfunction in obesity".Experimental and Clinical Endocrinology & Diabetes.117(6): 241–250.doi:10.1055/s-0029-1192044.PMID19358089.
- ^Kahn SE,Hull RL, Utzschneider KM (December 2006). "Mechanisms linking obesity to insulin resistance and type 2 diabetes".Nature.444(7121): 840–846.Bibcode:2006Natur.444..840K.doi:10.1038/nature05482.PMID17167471.S2CID120626.
- ^Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. (March 2006)."Recent advances in the relationship between obesity, inflammation, and insulin resistance".European Cytokine Network.17(1): 4–12.PMID16613757.
Several factors derived not only from adipocytes but also from infiltrated macrophages probably contribute to the pathogenesis of insulin resistance.
- ^Kahn BB, Flier JS (August 2000)."Obesity and insulin resistance".The Journal of Clinical Investigation.106(4): 473–481.doi:10.1172/JCI10842.PMC380258.PMID10953022.
- ^Rawson RB (August 2003). "The SREBP pathway--insights from Insigs and insects".Nature Reviews. Molecular Cell Biology.4(8): 631–640.doi:10.1038/nrm1174.PMID12923525.S2CID20818196.
- ^abCarobbio S, Hagen RM, Lelliott CJ, Slawik M, Medina-Gomez G, Tan CY, et al. (November 2013)."Adaptive changes of the Insig1/SREBP1/SCD1 set point help adipose tissue to cope with increased storage demands of obesity".Diabetes.62(11): 3697–3708.doi:10.2337/db12-1748.PMC3806615.PMID23919961.
- ^Nelson LR, Bulun SE (September 2001). "Estrogen production and action".Journal of the American Academy of Dermatology.45(3 Suppl): S116–S124.doi:10.1067/mjd.2001.117432.PMID11511861.
- ^"FERTILITY FACT: Female Risks".American Society for Reproductive Medicine (ASRM). Archived fromthe originalon 22 September 2007.
- ^Klok MD, Jakobsdottir S, Drent ML (January 2007)."The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review".Obesity Reviews.8(1): 21–34.doi:10.1111/j.1467-789X.2006.00270.x.PMID17212793.S2CID24266123.
External links
edit- Histology image: 08201loa– Histology Learning System at Boston University – "Connective Tissue: unilocular (white) adipocytes"
- Histology image: 04901lob– Histology Learning System at Boston University – "Connective Tissue: multilocular (brown) adipocytes"