Chemotherapy(often abbreviatedchemo,sometimesCTXandCTx) is the type ofcancer treatmentthat uses one or more anti-cancer drugs (chemotherapeutic agentsoralkylating agents) in a standardregimen.Chemotherapy may be given with acurativeintent (which almost always involves combinations of drugs), or it may aim only to prolong life or toreduce symptoms(palliativechemotherapy). Chemotherapy is one of the major categories of the medical discipline specifically devoted topharmacotherapyforcancer,which is calledmedical oncology.[1][2]
| Chemotherapy | |
|---|---|
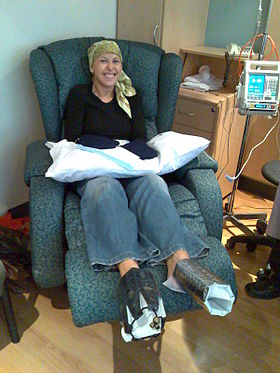 A woman being treated withdocetaxelchemotherapy forbreast cancer.Cold mittens and cold booties are placed on her hands and feet to reduce harm to her nails. | |
| Other names | chemo, CTX, CTx |
The termchemotherapynow means the non-specific use of intracellularpoisonsto inhibitmitosis(cell division) or to induceDNA damage(so thatDNA repaircan augment chemotherapy).[3]This meaning excludes the more-selective agents that block extracellular signals (signal transduction). Therapies with specific molecular or genetic targets, which inhibit growth-promoting signals from classic endocrine hormones (primarilyestrogensfor breast cancer andandrogensfor prostate cancer), are now calledhormonal therapies.Other inhibitions of growth-signals, such as those associated withreceptor tyrosine kinases,aretargeted therapy.
The use of drugs (whether chemotherapy, hormonal therapy, or targeted therapy) issystemic therapyfor cancer: they are introduced into the blood stream (the system) and therefore can treat cancer anywhere in the body. Systemic therapy is often used with other,local therapy(treatments that work only where they are applied), such asradiation,surgery,andhyperthermia.
Traditional chemotherapeutic agents arecytotoxicby means of interfering with cell division (mitosis) but cancer cells vary widely in their susceptibility to these agents. To a large extent, chemotherapy can be thought of as a way to damage or stress cells, which may then lead to cell death ifapoptosisis initiated. Many of the side effects of chemotherapy can be traced to damage to normal cells that divide rapidly and are thus sensitive to anti-mitotic drugs: cells in thebone marrow,digestive tractandhair follicles.This results in the most common side-effects of chemotherapy:myelosuppression(decreased production of blood cells, hence that alsoimmunosuppression),mucositis(inflammation of the lining of the digestive tract), andalopecia(hair loss). Because of the effect on immune cells (especially lymphocytes), chemotherapy drugs often find use in a host of diseases that result from harmful overactivity of the immune system against self (so-calledautoimmunity). These includerheumatoid arthritis,systemic lupus erythematosus,multiple sclerosis,vasculitisand many others.
Treatment strategies
editThere are a number of strategies in the administration of chemotherapeutic drugs used today. Chemotherapy may be given with acurativeintent or it may aim to prolong life or topalliate symptoms.
- Induction chemotherapy is the first line treatment of cancer with a chemotherapeutic drug. This type of chemotherapy is used for curative intent.[1][6]: 55–59
- Combined modality chemotherapy is the use of drugs with othercancer treatments,such assurgery,radiation therapy,orhyperthermia therapy.
- Consolidation chemotherapy is given after remission in order to prolong the overall disease-free time and improve overall survival. The drug that is administered is the same as the drug that achieved remission.[6]: 55–59
- Intensification chemotherapy is identical to consolidation chemotherapy but a different drug than the induction chemotherapy is used.[6]: 55–59
- Combination chemotherapyinvolves treating a person with a number of different drugs simultaneously. The drugs differ in their mechanism and side-effects. The biggest advantage is minimising the chances of resistance developing to any one agent. Also, the drugs can often be used at lower doses, reducing toxicity.[6]: 55–59 [7]: 17–18 [5]
- Neoadjuvantchemotherapy is given prior to a local treatment such as surgery, and is designed to shrink the primary tumor.[6]: 55–59 It is also given for cancers with a high risk of micrometastatic disease.[8]: 42
- Adjuvant chemotherapyis given after a local treatment (radiotherapy or surgery). It can be used when there is little evidence of cancer present, but there is risk of recurrence.[6]: 55–59 It is also useful in killing any cancerous cells that have spread to other parts of the body. Thesemicrometastasescan be treated with adjuvant chemotherapy and can reduce relapse rates caused by these disseminated cells.[9]
- Maintenance chemotherapy is a repeated low-dose treatment to prolong remission.[5][6]: 55–59
- Salvage chemotherapy or palliative chemotherapy is given without curative intent, but simply to decrease tumor load and increase life expectancy. For these regimens, in general, a better toxicity profile is expected.[6]: 55–59
Allchemotherapy regimensrequire that the recipient be capable of undergoing the treatment.Performance statusis often used as a measure to determine whether a person can receive chemotherapy, or whether dose reduction is required. Because only a fraction of the cells in a tumor die with each treatment (fractional kill), repeated doses must be administered to continue to reduce the size of the tumor.[10]Current chemotherapy regimens apply drug treatment in cycles, with the frequency and duration of treatments limited by toxicity.[11]
Effectiveness
editThe effectiveness of chemotherapy depends on the type of cancer and the stage. The overall effectiveness ranges from being curative for some cancers, such as someleukemias,[12][13]to being ineffective, such as in somebrain tumors,[14]to being needless in others, like mostnon-melanoma skin cancers.[15]
Dosage
editDosage of chemotherapy can be difficult: If the dose is too low, it will be ineffective against the tumor, whereas, at excessive doses, the toxicity (side-effects) will be intolerable to the person receiving it.[4]The standard method of determining chemotherapy dosage is based on calculatedbody surface area(BSA). The BSA is usually calculated with a mathematical formula or anomogram,using the recipient's weight and height, rather than by direct measurement of body area. This formula was originally derived in a 1916 study and attempted to translate medicinal doses established with laboratory animals to equivalent doses for humans.[16]The study only included nine human subjects.[17]When chemotherapy was introduced in the 1950s, the BSA formula was adopted as the official standard for chemotherapy dosing for lack of a better option.[18][19]
The validity of this method in calculating uniform doses has been questioned because the formula only takes into account the individual's weight and height. Drug absorption and clearance are influenced by multiple factors, including age, sex, metabolism, disease state, organ function, drug-to-drug interactions, genetics, and obesity, which have major impacts on the actual concentration of the drug in the person's bloodstream.[18][20][21]As a result, there is high variability in the systemic chemotherapy drug concentration in people dosed by BSA, and this variability has been demonstrated to be more than ten-fold for many drugs.[17][22]In other words, if two people receive the same dose of a given drug based on BSA, the concentration of that drug in the bloodstream of one person may be 10 times higher or lower compared to that of the other person.[22]This variability is typical with many chemotherapy drugs dosed by BSA, and, as shown below, was demonstrated in a study of 14 common chemotherapy drugs.[17]
The result of this pharmacokinetic variability among people is that many people do not receive the right dose to achieve optimal treatment effectiveness with minimized toxic side effects. Some people are overdosed while others are underdosed.[18][20][21][23][24][25][26]For example, in a randomized clinical trial, investigators found 85% of metastatic colorectal cancer patients treated with 5-fluorouracil (5-FU) did not receive the optimal therapeutic dose when dosed by the BSA standard—68% were underdosed and 17% were overdosed.[23]
There has been controversy over the use of BSA to calculate chemotherapy doses for people who areobese.[27]Because of their higher BSA, clinicians often arbitrarily reduce the dose prescribed by the BSA formula for fear ofoverdosing.[27]In many cases, this can result in sub-optimal treatment.[27]
Several clinical studies have demonstrated that when chemotherapy dosing is individualized to achieve optimal systemic drug exposure, treatment outcomes are improved and toxic side effects are reduced.[23][25]In the 5-FU clinical study cited above, people whose dose was adjusted to achieve a pre-determined target exposure realized an 84% improvement in treatment response rate and a six-month improvement in overall survival (OS) compared with those dosed by BSA.[23]
In the same study, investigators compared the incidence of common 5-FU-associated grade 3/4 toxicities between the dose-adjusted people and people dosed per BSA.[23]The incidence of debilitating grades of diarrhea was reduced from 18% in the BSA-dosed group to 4% in the dose-adjusted group and serious hematologic side effects were eliminated.[23]Because of the reduced toxicity, dose-adjusted patients were able to be treated for longer periods of time.[23]BSA-dosed people were treated for a total of 680 months while people in the dose-adjusted group were treated for a total of 791 months.[23]Completing the course of treatment is an important factor in achieving better treatment outcomes.
Similar results were found in a study involving people with colorectal cancer who have been treated with the popularFOLFOXregimen.[25]The incidence of serious diarrhea was reduced from 12% in the BSA-dosed group of patients to 1.7% in the dose-adjusted group, and the incidence of severe mucositis was reduced from 15% to 0.8%.[25]
The FOLFOX study also demonstrated an improvement in treatment outcomes.[25]Positive response increased from 46% in the BSA-dosed group to 70% in the dose-adjusted group. Median progression free survival (PFS) and overall survival (OS) both improved by six months in the dose adjusted group.[25]
One approach that can help clinicians individualize chemotherapy dosing is to measure the drug levels in blood plasma over time and adjust dose according to a formula or algorithm to achieve optimal exposure. With an established target exposure for optimized treatment effectiveness with minimized toxicities, dosing can be personalized to achieve target exposure and optimal results for each person. Such an algorithm was used in the clinical trials cited above and resulted in significantly improved treatment outcomes.[28]
Oncologists are already individualizing dosing of some cancer drugs based on exposure.Carboplatin[29]: 4 andbusulfan[30][31]dosing rely upon results from blood tests to calculate the optimal dose for each person. Simple blood tests are also available for dose optimization ofmethotrexate,[32]5-FU,paclitaxel,anddocetaxel.[33][34]
The serum albumin level immediately prior to chemotherapy administration is an independent prognostic predictor of survival in various cancer types.[35]
Types
editAlkylating agents
editAlkylating agents are the oldest group of chemotherapeutics in use today. Originally derived frommustard gasused inWorld War I,there are now many types of alkylating agents in use.[4]They are so named because of their ability toalkylatemany molecules, includingproteins,RNAandDNA.This ability to bindcovalentlyto DNA via theiralkyl groupis the primary cause for their anti-cancer effects.[37]DNA is made of two strands and the molecules may either bind twice to one strand of DNA (intrastrand crosslink) or may bind once to both strands (interstrand crosslink). If the cell tries to replicate crosslinked DNA duringcell division,or tries to repair it, the DNA strands can break. This leads to a form of programmed cell death calledapoptosis.[36][38]Alkylating agents will work at any point in the cell cycle and thus are known as cell cycle-independent drugs. For this reason, the effect on the cell is dose dependent; the fraction of cells that die is directly proportional to the dose of drug.[39]
The subtypes of alkylating agents are thenitrogen mustards,nitrosoureas,tetrazines,aziridines,[40]cisplatinsand derivatives, and non-classical alkylating agents. Nitrogen mustards includemechlorethamine,cyclophosphamide,melphalan,chlorambucil,ifosfamideandbusulfan.Nitrosoureas includeN-Nitroso-N-methylurea(MNU),carmustine(BCNU),lomustine(CCNU) andsemustine(MeCCNU),fotemustineandstreptozotocin.Tetrazines includedacarbazine,mitozolomideandtemozolomide.Aziridines includethiotepa,mytomycinand diaziquone (AZQ). Cisplatin and derivatives includecisplatin,carboplatinandoxaliplatin.[37][38]They impair cell function by formingcovalent bondswith theamino,carboxyl,sulfhydryl,andphosphate groupsin biologically important molecules.[41]Non-classical alkylating agents includeprocarbazineand hexamethylmelamine.[37][38]
Antimetabolites
editAnti-metabolitesare a group of molecules that impede DNA and RNA synthesis. Many of them have a similar structure to the building blocks of DNA and RNA. The building blocks arenucleotides;a molecule comprising anucleobase,a sugar and aphosphate group.The nucleobases are divided intopurines(guanineandadenine) andpyrimidines(cytosine,thymineanduracil). Anti-metabolites resemble either nucleobases or nucleosides (a nucleotide without the phosphate group), but have alteredchemical groups.[42]These drugs exert their effect by either blocking the enzymes required for DNA synthesis or becoming incorporated into DNA or RNA. By inhibiting the enzymes involved in DNA synthesis, they prevent mitosis because the DNA cannot duplicate itself. Also, after misincorporation of the molecules into DNA,DNA damagecan occur and programmed cell death (apoptosis) is induced. Unlike alkylating agents, anti-metabolites are cell cycle dependent. This means that they only work during a specific part of the cell cycle, in this caseS-phase(the DNA synthesis phase). For this reason, at a certain dose, the effect plateaus and proportionally no more cell death occurs with increased doses. Subtypes of the anti-metabolites are theanti-folates,fluoropyrimidines, deoxynucleoside analogues andthiopurines.[37][42]
The anti-folates includemethotrexateandpemetrexed.Methotrexate inhibitsdihydrofolate reductase(DHFR), an enzyme that regeneratestetrahydrofolatefromdihydrofolate.When the enzyme is inhibited by methotrexate, the cellular levels of folate coenzymes diminish. These are required forthymidylateand purine production, which are both essential for DNA synthesis and cell division.[6]: 55–59 [7]: 11 Pemetrexed is another anti-metabolite that affects purine and pyrimidine production, and therefore also inhibits DNA synthesis. It primarily inhibits the enzymethymidylate synthase,but also has effects on DHFR, aminoimidazole carboxamide ribonucleotide formyltransferase andglycinamide ribonucleotide formyltransferase.[43]The fluoropyrimidines includefluorouracilandcapecitabine.Fluorouracil is a nucleobase analogue that is metabolised in cells to form at least two active products; 5-fluourouridine monophosphate (FUMP) and 5-fluoro-2'-deoxyuridine 5'-phosphate (fdUMP). FUMP becomes incorporated into RNA and fdUMP inhibits the enzyme thymidylate synthase; both of which lead to cell death.[7]: 11 Capecitabine is aprodrugof 5-fluorouracil that is broken down in cells to produce the active drug.[44]The deoxynucleoside analogues includecytarabine,gemcitabine,decitabine,azacitidine,fludarabine,nelarabine,cladribine,clofarabine,andpentostatin.The thiopurines includethioguanineandmercaptopurine.[37][42]
Anti-microtubule agents
editAnti-microtubule agentsareplant-derived chemicals that block cell division by preventingmicrotubulefunction. Microtubules are an important cellular structure composed of two proteins,α-tubulinandβ-tubulin.They are hollow, rod-shaped structures that are required for cell division, among other cellular functions.[45]Microtubules are dynamic structures, which means that they are permanently in a state of assembly and disassembly.Vincaalkaloidsandtaxanesare the two main groups of anti-microtubule agents, and although both of these groups of drugs cause microtubule dysfunction, their mechanisms of action are completely opposite:Vincaalkaloids prevent the assembly of microtubules, whereas taxanes prevent their disassembly. By doing so, they can inducemitotic catastrophein the cancer cells.[46]Following this, cell cycle arrest occurs, which induces programmed cell death (apoptosis).[37][47]These drugs can also affectblood vessel growth,an essential process that tumours utilise in order to grow and metastasise.[47]
Vincaalkaloids are derived from theMadagascar periwinkle,Catharanthus roseus,[48][49]formerly known asVinca rosea.They bind to specific sites on tubulin, inhibiting the assembly of tubulin into microtubules. The originalvincaalkaloids arenatural productsthat includevincristineandvinblastine.[50][51][52][53]Following the success of these drugs, semi-syntheticvincaalkaloids were produced:vinorelbine(used in the treatment ofnon-small-cell lung cancer[52][54][55]),vindesine,andvinflunine.[47]These drugs arecell cycle-specific. They bind to the tubulin molecules inS-phaseand prevent proper microtubule formation required forM-phase.[39]
Taxanes are natural and semi-synthetic drugs. The first drug of their class,paclitaxel,was originally extracted fromTaxus brevifolia,the Pacific yew. Now this drug and another in this class,docetaxel,are produced semi-synthetically from a chemical found in the bark of another yew tree,Taxus baccata.[56]
Podophyllotoxinis an antineoplasticlignanobtained primarily from theAmerican mayapple(Podophyllum peltatum) andHimalayan mayapple(Sinopodophyllum hexandrum). It has anti-microtubule activity, and its mechanism is similar to that ofvincaalkaloids in that they bind to tubulin, inhibiting microtubule formation. Podophyllotoxin is used to produce two other drugs with different mechanisms of action:etoposideandteniposide.[57][58]
Topoisomerase inhibitors
editTopoisomerase inhibitors are drugs that affect the activity of two enzymes:topoisomerase Iandtopoisomerase II.When the DNA double-strand helix is unwound, during DNA replication ortranscription,for example, the adjacent unopened DNA winds tighter (supercoils), like opening the middle of a twisted rope. The stress caused by this effect is in part aided by the topoisomerase enzymes. They produce single- or double-strand breaks into DNA, reducing the tension in the DNA strand. This allows the normal unwinding of DNA to occur duringreplicationor transcription. Inhibition of topoisomerase I or II interferes with both of these processes.[59][60]
Two topoisomerase I inhibitors,irinotecanandtopotecan,are semi-synthetically derived fromcamptothecin,which is obtained from the Chinese ornamental treeCamptotheca acuminata.[39]Drugs that target topoisomerase II can be divided into two groups. The topoisomerase II poisons cause increased levels enzymes bound to DNA. This prevents DNA replication and transcription, causes DNA strand breaks, and leads to programmed cell death (apoptosis). These agents includeetoposide,doxorubicin,mitoxantroneandteniposide.The second group, catalytic inhibitors, are drugs that block the activity of topoisomerase II, and therefore prevent DNA synthesis and translation because the DNA cannot unwind properly. This group includesnovobiocin,merbarone, andaclarubicin,which also have other significant mechanisms of action.[61]
Cytotoxic antibiotics
editThe cytotoxicantibioticsare a varied group of drugs that have various mechanisms of action. The common theme that they share in their chemotherapy indication is that they interruptcell division.The most important subgroup is theanthracyclinesand thebleomycins;other prominent examples includemitomycin Candactinomycin.[62]
Among the anthracyclines,doxorubicinanddaunorubicinwere the first, and were obtained from thebacteriumStreptomyces peucetius.[63]Derivatives of these compounds includeepirubicinandidarubicin.Other clinically used drugs in the anthracycline group arepirarubicin,aclarubicin,andmitoxantrone.[64]The mechanisms of anthracyclines includeDNA intercalation(molecules insert between the two strands of DNA), generation of highly reactivefree radicalsthat damage intercellular molecules and topoisomerase inhibition.[65]
Actinomycin is a complex molecule that intercalates DNA and preventsRNA synthesis.[66]
Bleomycin, aglycopeptideisolated fromStreptomyces verticillus,also intercalates DNA, but producesfree radicalsthat damage DNA. This occurs when bleomycin binds to ametal ion,becomeschemically reducedand reacts withoxygen.[67][6]: 87
Mitomycin is a cytotoxic antibiotic with the ability to alkylate DNA.[68]
Delivery
editMost chemotherapy isdeliveredintravenously,although a number of agents can be administered orally (e.g.,melphalan,busulfan,capecitabine). According to a recent (2016) systematic review, oral therapies present additional challenges for patients and care teams to maintain and support adherence to treatment plans.[69]
There are many intravenous methods of drug delivery, known as vascular access devices. These include thewinged infusion device,peripheral venous catheter,midline catheter,peripherally inserted central catheter(PICC),central venous catheterandimplantable port.The devices have different applications regarding duration of chemotherapy treatment, method of delivery and types of chemotherapeutic agent.[7]: 94–95
Depending on the person, the cancer, the stage of cancer, the type of chemotherapy, and the dosage, intravenous chemotherapy may be given on either aninpatientor anoutpatientbasis. For continuous, frequent or prolonged intravenous chemotherapy administration, various systems may be surgically inserted into the vasculature to maintain access.[7]: 113–118 Commonly used systems are theHickman line,thePort-a-Cath,and thePICC line.These have a lower infection risk, are much less prone tophlebitisorextravasation,and eliminate the need for repeated insertion of peripheral cannulae.[70]
Isolated limb perfusion(often used inmelanoma),[71]or isolated infusion of chemotherapy into the liver[72]or the lung have been used to treat some tumors. The main purpose of these approaches is to deliver a very high dose of chemotherapy to tumor sites without causing overwhelming systemic damage.[73]These approaches can help control solitary or limited metastases, but they are by definition not systemic, and, therefore, do not treat distributed metastases ormicrometastases.[citation needed]
Topical chemotherapies, such as5-fluorouracil,are used to treat some cases ofnon-melanoma skin cancer.[74]
If the cancer hascentral nervous systeminvolvement, or with meningeal disease,intrathecalchemotherapy may be administered.[4]
Adverse effects
editChemotherapeutic techniques have a range of side effects that depend on the type of medications used. The most common medications affect mainly thefast-dividing cellsof the body, such as blood cells and the cells lining the mouth, stomach, and intestines. Chemotherapy-related toxicities can occur acutely after administration, within hours or days, or chronically, from weeks to years.[6]: 265
Immunosuppression and myelosuppression
editVirtually all chemotherapeutic regimens can cause depression of theimmune system,often by paralysing thebone marrowand leading to a decrease ofwhite blood cells,red blood cells,andplatelets. Anemiaandthrombocytopeniamay requireblood transfusion.Neutropenia(a decrease of theneutrophil granulocytecount below 0.5 x 109/litre) can be improved with syntheticG-CSF(granulocyte-colony-stimulating factor, e.g.,filgrastim,lenograstim,efbemalenograstim alfa).[75]
In very severemyelosuppression,which occurs in some regimens, almost all the bone marrowstem cells(cells that producewhiteandred blood cells) are destroyed, meaningallogenicorautologousbone marrow cell transplantsare necessary. (In autologous BMTs, cells are removed from the person before the treatment, multiplied and then re-injected afterward; inallogenicBMTs, the source is a donor.) However, some people still develop diseases because of this interference with bone marrow.[76]
Although people receiving chemotherapy are encouraged to wash their hands, avoid sick people, and take other infection-reducing steps, about 85% of infections are due to naturally occurring microorganisms in the person's owngastrointestinal tract(includingoral cavity) and skin.[77]: 130 This may manifest as systemic infections, such assepsis,or as localized outbreaks, such asHerpes simplex,shingles,or other members of theHerpesviridea.[78]The risk of illness and death can be reduced by taking common antibiotics such asquinolonesortrimethoprim/sulfamethoxazolebefore any fever or sign of infection appears.[79]Quinolones show effective prophylaxis mainly with hematological cancer.[79]However, in general, for every five people who are immunosuppressed following chemotherapy who take an antibiotic, one fever can be prevented; for every 34 who take an antibiotic, one death can be prevented.[79]Sometimes, chemotherapy treatments are postponed because the immune system is suppressed to a critically low level.[citation needed]
InJapan,the government has approved the use of somemedicinal mushroomslikeTrametes versicolor,to counteract depression of the immune system in people undergoing chemotherapy.[80]
Trilaciclibis an inhibitor ofcyclin-dependent kinase4/6 approved for the prevention of myelosuppression caused by chemotherapy. The drug is given before chemotherapy to protect bone marrow function.[81]
Neutropenic enterocolitis
editDue to immune system suppression,neutropenic enterocolitis(typhlitis) is a "life-threatening gastrointestinal complication of chemotherapy."[82]Typhlitisis an intestinal infection which may manifest itself through symptoms includingnausea,vomiting,diarrhea,adistended abdomen,fever,chills,orabdominal painand tenderness.[83]
Typhlitisis amedical emergency.It has a very poorprognosisand is often fatal unless promptly recognized and aggressively treated.[84]Successful treatment hinges on early diagnosis provided by a high index of suspicion and the use of CT scanning, nonoperative treatment for uncomplicated cases, and sometimes elective righthemicolectomyto prevent recurrence.[84]
Gastrointestinal distress
editNausea,vomiting,anorexia,diarrhea,abdominal cramps, andconstipationare common side-effects of chemotherapeutic medications that kill fast-dividing cells.[85]Malnutritionanddehydrationcan result when the recipient does not eat or drink enough, or when the person vomits frequently, because of gastrointestinal damage. This can result in rapid weight loss, or occasionally in weight gain, if the person eats too much in an effort to allay nausea or heartburn. Weight gain can also be caused by some steroid medications. These side-effects can frequently be reduced or eliminated withantiemeticdrugs. Low-certainty evidence also suggests that probiotics may have a preventative and treatment effect of diarrhoea related to chemotherapy alone and with radiotherapy.[86]However, a high index of suspicion is appropriate, since diarrhoea and bloating are also symptoms oftyphlitis,a very serious and potentially life-threateningmedical emergencythat requires immediate treatment.[87]
Anemia
editAnemiacan be a combined outcome caused by myelosuppressive chemotherapy, and possible cancer-related causes such asbleeding,blood celldestruction (hemolysis), hereditary disease, kidney dysfunction, nutritional deficiencies oranemia of chronic disease.Treatments to mitigate anemia include hormones to boost blood production (erythropoietin),iron supplements,andblood transfusions.[88][89][90]Myelosuppressive therapy can cause a tendency to bleed easily, leading to anemia. Medications that kill rapidly dividing cells or blood cells can reduce the number ofplateletsin the blood, which can result inbruisesandbleeding.Extremely low platelet counts may be temporarily boosted throughplatelet transfusionsand new drugs to increase platelet counts during chemotherapy are being developed.[91][92][93][94]Sometimes, chemotherapy treatments are postponed to allow platelet counts to recover.
Fatiguemay be a consequence of the cancer or its treatment, and can last for months to years after treatment. One physiological cause of fatigue is anemia, which can be caused by chemotherapy,surgery,radiotherapy,primary and metastatic disease or nutritional depletion.[95][96]Aerobic exercisehas been found to be beneficial in reducing fatigue in people withsolid tumours.[97]
Nausea and vomiting
editNauseaandvomitingare two of the most feared cancer treatment-related side-effects for people with cancer and their families. In 1983, Coates et al. found that people receiving chemotherapy ranked nausea and vomiting as the first and second most severe side-effects, respectively.[98]Up to 20% of people receiving highly emetogenic agents in this era postponed, or even refused potentially curative treatments.[99]Chemotherapy-induced nausea and vomiting (CINV) are common with many treatments and some forms of cancer. Since the 1990s, several novel classes ofantiemeticshave been developed and commercialized, becoming a nearly universal standard in chemotherapy regimens, and helping to successfully manage these symptoms in many people. Effective mediation of these unpleasant and sometimes debilitating symptoms results in increased quality of life for the recipient and more efficient treatment cycles, due to less stoppage of treatment due to better tolerance and better overall health.[100]
Hair loss
editHair loss(alopecia) can be caused by chemotherapy that kills rapidly dividing cells; other medications may cause hair to thin. These are most often temporary effects: hair usually starts to regrow a few weeks after the last treatment, but sometimes with a change in color, texture, thickness or style. Sometimes hair has a tendency to curl after regrowth, resulting in "chemo curls." Severe hair loss occurs most often with drugs such asdoxorubicin,daunorubicin,paclitaxel,docetaxel,cyclophosphamide,ifosfamideandetoposide.Permanent thinning or hair loss can result from some standard chemotherapy regimens.[101]
Chemotherapy induced hair loss occurs by a non-androgenic mechanism, and can manifest asalopecia totalis,telogen effluvium, or less oftenalopecia areata.[102]It is usually associated with systemic treatment due to the high mitotic rate of hair follicles, and more reversible than androgenic hair loss,[103][104]although permanent cases can occur.[105]Chemotherapy induces hair loss in women more often than men.[106]
Scalp coolingoffers a means of preventing both permanent and temporary hair loss; however, concerns about this method have been raised.[107][108]
Secondary neoplasm
editDevelopment of secondary neoplasia after successful chemotherapy or radiotherapy treatment can occur. The most commonsecondary neoplasmis secondary acute myeloid leukemia, which develops primarily after treatment with alkylating agents or topoisomerase inhibitors.[109]Survivors ofchildhood cancerare more than 13 times as likely to get asecondary neoplasmduring the 30 years after treatment than the general population.[110]Not all of this increase can be attributed to chemotherapy.
Infertility
editSome types of chemotherapy are gonadotoxic and may causeinfertility.[111]Chemotherapies with high risk include procarbazine and other alkylating drugs such as cyclophosphamide, ifosfamide, busulfan, melphalan, chlorambucil, and chlormethine.[111]Drugs with medium risk include doxorubicin and platinum analogs such as cisplatin and carboplatin.[111]On the other hand, therapies with low risk of gonadotoxicity include plant derivatives such as vincristine and vinblastine,antibioticssuch as bleomycin and dactinomycin, and antimetabolites such as methotrexate, mercaptopurine, and 5-fluorouracil.[111]
Female infertilityby chemotherapy appears to be secondary topremature ovarian failureby loss ofprimordial follicles.[112]This loss is not necessarily a direct effect of the chemotherapeutic agents, but could be due to an increased rate of growth initiation to replace damaged developing follicles.[112]
People may choose between several methods offertility preservationprior to chemotherapy, includingcryopreservationof semen, ovarian tissue, oocytes, or embryos.[113]As more than half of cancer patients are elderly, this adverse effect is only relevant for a minority of patients. A study in France between 1999 and 2011 came to the result that embryo freezing before administration of gonadotoxic agents to females caused a delay of treatment in 34% of cases, and a live birth in 27% of surviving cases who wanted to become pregnant, with the follow-up time varying between 1 and 13 years.[114]
Potential protective or attenuating agents includeGnRH analogs,where several studies have shown a protective effectin vivoin humans, but some studies show no such effect.Sphingosine-1-phosphate(S1P) has shown similar effect, but its mechanism of inhibiting thesphingomyelin apoptotic pathwaymay also interfere with theapoptosisaction of chemotherapy drugs.[115]
In chemotherapy as aconditioning regimenin hematopoietic stem cell transplantation, a study of people conditioned with cyclophosphamide alone for severe aplastic anemia came to the result that ovarian recovery occurred in all women younger than 26 years at time of transplantation, but only in five of 16 women older than 26 years.[116]
Teratogenicity
editChemotherapy isteratogenicduringpregnancy,especially during thefirst trimester,to the extent thatabortionusually is recommended if pregnancy in this period is found during chemotherapy.[117]Second- and third-trimester exposure does not usually increase the teratogenic risk and adverse effects on cognitive development, but it may increase the risk of variouscomplications of pregnancyand fetal myelosuppression.[117]
Female patients of reproductive potential should use effectivecontraceptionduring chemotherapy and for a few months after the last dose (e.g. 6 month fordoxorubicin[118]).
In males previously having undergone chemotherapy or radiotherapy, there appears to be no increase in genetic defects or congenital malformations in their children conceived after therapy.[117]The use ofassisted reproductive technologiesandmicromanipulation techniquesmight increase this risk.[117]In females previously having undergone chemotherapy, miscarriage and congenital malformations are not increased in subsequent conceptions.[117]However, whenin vitro fertilizationandembryo cryopreservationis practised between or shortly after treatment, possible genetic risks to the growing oocytes exist, and hence it has been recommended that the babies be screened.[117]
Peripheral neuropathy
editBetween 30 and 40 percent of people undergoing chemotherapy experiencechemotherapy-induced peripheral neuropathy(CIPN), a progressive, enduring, and often irreversible condition, causing pain, tingling, numbness and sensitivity to cold, beginning in the hands and feet and sometimes progressing to the arms and legs.[119]Chemotherapy drugs associated with CIPN includethalidomide,epothilones,vincaalkaloids, taxanes,proteasome inhibitors,and the platinum-based drugs.[119][120]Whether CIPN arises, and to what degree, is determined by the choice of drug, duration of use, the total amount consumed and whether the person already hasperipheral neuropathy.Though the symptoms are mainly sensory, in some casesmotor nervesand theautonomic nervous systemare affected.[121]CIPN often follows the first chemotherapy dose and increases in severity as treatment continues, but this progression usually levels off at completion of treatment. The platinum-based drugs are the exception; with these drugs, sensation may continue to deteriorate for several months after the end of treatment.[122]Some CIPN appears to be irreversible.[122]Pain can often be managed with drug or other treatment but the numbness is usually resistant to treatment.[123]
Cognitive impairment
editSome people receiving chemotherapy report fatigue or non-specific neurocognitive problems, such as an inability to concentrate; this is sometimes calledpost-chemotherapy cognitive impairment,referred to as "chemo brain" in popular and social media.[124]
Tumor lysis syndrome
editIn particularly large tumors and cancers with highwhite cell counts,such aslymphomas,teratomas,and someleukemias,some people developtumor lysis syndrome.The rapid breakdown of cancer cells causes the release of chemicals from the inside of the cells. Following this, high levels ofuric acid,potassiumandphosphateare found in the blood. High levels of phosphate induce secondary hypoparathyroidism, resulting in low levels of calcium in the blood.[125]This causes kidney damage and the high levels of potassium can causecardiac arrhythmia.Although prophylaxis is available and is often initiated in people with large tumors, this is a dangerous side-effect that can lead to death if left untreated.[7]: 202
Organ damage
editCardiotoxicity(heart damage) is especially prominent with the use ofanthracyclinedrugs (doxorubicin,epirubicin,idarubicin,andliposomal doxorubicin). The cause of this is most likely due to the production offree radicalsin the cell and subsequentDNA damage.Other chemotherapeutic agents that cause cardiotoxicity, but at a lower incidence, arecyclophosphamide,docetaxelandclofarabine.[126]
Hepatotoxicity(liver damage) can be caused by many cytotoxic drugs. The susceptibility of an individual to liver damage can be altered by other factors such as the cancer itself,viral hepatitis,immunosuppressionandnutritional deficiency.The liver damage can consist of damage to liver cells,hepatic sinusoidal syndrome(obstruction of the veins in the liver),cholestasis(where bile does not flow from the liver to the intestine) andliver fibrosis.[127][128]
Nephrotoxicity(kidney damage) can be caused bytumor lysis syndromeand also due direct effects of drug clearance by the kidneys. Different drugs will affect different parts of the kidney and the toxicity may beasymptomatic(only seen on blood or urine tests) or may causeacute kidney injury.[129][130]
Ototoxicity(damage to the inner ear) is a common side effect of platinum based drugs that can produce symptoms such as dizziness andvertigo.[131][132]Children treated with platinum analogues have been found to be at risk for developing hearing loss.[133][134][135]
Other side-effects
editLess common side-effects include red skin (erythema), dry skin, damaged fingernails, a dry mouth (xerostomia),water retention,andsexual impotence.Some medications can triggerallergicorpseudoallergicreactions.
Specific chemotherapeutic agents are associated with organ-specific toxicities, includingcardiovascular disease(e.g.,doxorubicin),interstitial lung disease(e.g.,bleomycin) and occasionallysecondary neoplasm(e.g.,MOPPtherapy for Hodgkin's disease).[136]
Hand-foot syndromeis another side effect to cytotoxic chemotherapy.[137]
Nutritional problems are also frequently seen in cancer patients at diagnosis and through chemotherapy treatment. Research suggests that in children and young people undergoing cancer treatment,parenteral nutritionmay help with this leading to weight gain and increased calorie and protein intake, when compared to enteral nutrition.[138]
Limitations
editChemotherapy does not always work, and even when it is useful, it may not completely destroy the cancer. People frequently fail to understand its limitations. In one study of people who had been newly diagnosed with incurable,stage 4 cancer,more than two-thirds of people with lung cancer and more than four-fifths of people with colorectal cancer still believed that chemotherapy was likely to cure their cancer.[139]
Theblood–brain barrierposes an obstacle to delivery of chemotherapy to thebrain.This is because the brain has an extensive system in place to protect it from harmful chemicals. Drug transporters can pump out drugs from the brain and brain's blood vessel cells into thecerebrospinal fluidand blood circulation. These transporters pump out most chemotherapy drugs, which reduces their efficacy for treatment of brain tumors. Only smalllipophilicalkylating agentssuch aslomustineortemozolomideare able to cross this blood–brain barrier.[140][141][142]
Blood vesselsin tumors are very different from those seen in normal tissues. As a tumor grows, tumor cells furthest away from the blood vessels become low in oxygen (hypoxic). To counteract this they then signal for new blood vessels to grow. The newly formed tumor vasculature is poorly formed and does not deliver an adequate blood supply to all areas of the tumor. This leads to issues with drug delivery because many drugs will be delivered to the tumor by thecirculatory system.[143]
Resistance
editResistanceis a major cause of treatment failure in chemotherapeutic drugs. There are a few possible causes of resistance in cancer, one of which is the presence of small pumps on the surface of cancer cells that actively move chemotherapy from inside the cell to the outside. Cancer cells produce high amounts of these pumps, known asp-glycoprotein,in order to protect themselves from chemotherapeutics. Research on p-glycoprotein and other such chemotherapy efflux pumps is currently ongoing. Medications to inhibit the function of p-glycoprotein are undergoing investigation, but due to toxicities and interactions with anti-cancer drugs their development has been difficult.[144][145]Another mechanism of resistance isgene amplification,a process in which multiple copies of a gene are produced by cancer cells. This overcomes the effect of drugs that reduce the expression of genes involved in replication. With more copies of the gene, the drug can not prevent all expression of the gene and therefore the cell can restore its proliferative ability. Cancer cells can also cause defects in the cellular pathways ofapoptosis(programmed cell death). As most chemotherapy drugs kill cancer cells in this manner, defective apoptosis allows survival of these cells, making them resistant. Many chemotherapy drugs also cause DNA damage, which can be repaired byenzymesin the cell that carry outDNA repair.Upregulation of these genes can overcome the DNA damage and prevent the induction of apoptosis. Mutations in genes that produce drug target proteins, such astubulin,can occur which prevent the drugs from binding to the protein, leading to resistance to these types of drugs.[146]Drugs used in chemotherapy can induce cell stress, which can kill a cancer cell; however, under certain conditions, cells stress can induce changes in gene expression that enables resistance to several types of drugs.[147]Inlung cancer,the transcription factorNFκBis thought to play a role in resistance to chemotherapy, via inflammatory pathways.[148][149][150]
Cytotoxics and targeted therapies
editTargeted therapiesare a relatively new class of cancer drugs that can overcome many of the issues seen with the use of cytotoxics. They are divided into two groups: small molecule and antibodies. The massive toxicity seen with the use of cytotoxics is due to the lack of cell specificity of the drugs. They will kill any rapidly dividing cell, tumor or normal. Targeted therapies are designed to affect cellular proteins or processes that are utilised by the cancer cells.[151]This allows a high dose to cancer tissues with a relatively low dose to other tissues. Although theside effectsare often less severe than that seen of cytotoxic chemotherapeutics, life-threatening effects can occur. Initially, the targeted therapeutics were supposed to be solely selective for one protein. Now it is clear that there is often a range of protein targets that the drug can bind. An example target for targeted therapy is the BCR-ABL1 protein produced from thePhiladelphia chromosome,a genetic lesion found commonly inchronic myelogenous leukemiaand in some patients withacute lymphoblastic leukemia.Thisfusion proteinhas enzyme activity that can be inhibited byimatinib,asmall moleculedrug.[152][153][154][155]
Mechanism of action
editCanceris the uncontrolled growth ofcellscoupled withmalignantbehaviour: invasion andmetastasis(among other features).[156]It is caused by the interaction betweengeneticsusceptibility and environmental factors.[157][158]These factors lead to accumulations ofgenetic mutationsinoncogenes(genes that control the growth rate of cells) andtumor suppressor genes(genes that help to prevent cancer), which gives cancer cells their malignant characteristics, such as uncontrolled growth.[159]: 93–94
In the broad sense, most chemotherapeutic drugs work by impairingmitosis(cell division), effectively targetingfast-dividing cells.As these drugs cause damage to cells, they are termedcytotoxic.They prevent mitosis by various mechanisms including damaging DNA and inhibition of the cellular machinery involved in cell division.[39][160]One theory as to why these drugs kill cancer cells is that they induce a programmed form of cell death known asapoptosis.[161]
As chemotherapy affects cell division, tumors with highgrowth rates(such asacute myelogenous leukemiaand the aggressivelymphomas,includingHodgkin's disease) are more sensitive to chemotherapy, as a larger proportion of the targeted cells are undergoingcell divisionat any time. Malignancies with slower growth rates, such as indolent lymphomas, tend to respond to chemotherapy much more modestly.[4]Heterogeneic tumoursmay also display varying sensitivities to chemotherapy agents, depending on the subclonal populations within the tumor.[162]
Cells from theimmune systemalso make crucial contributions to the antitumor effects of chemotherapy.[163]For example, the chemotherapeutic drugsoxaliplatinandcyclophosphamidecan cause tumor cells to die in a way that is detectable by the immune system (calledimmunogenic cell death), which mobilizes immune cells with antitumor functions.[164]Chemotherapeutic drugs that cause cancer immunogenic tumor cell death can make unresponsive tumors sensitive toimmune checkpointtherapy.[165]
Other uses
editSome chemotherapy drugs are used in diseases other than cancer, such as in autoimmune disorders,[166]and noncancerousplasma cell dyscrasia.In some cases they are often used at lower doses, which means that the side effects are minimized,[166]while in other cases doses similar to ones used to treat cancer are used.Methotrexateis used in the treatment ofrheumatoid arthritis(RA),[167]psoriasis,[168]ankylosing spondylitis[169]andmultiple sclerosis.[170][171]The anti-inflammatory response seen in RA is thought to be due to increases inadenosine,which causesimmunosuppression;effects on immuno-regulatorycyclooxygenase-2 enzyme pathways; reduction in pro-inflammatorycytokines;and anti-proliferative properties.[167]Although methotrexate is used to treat both multiple sclerosis and ankylosing spondylitis, its efficacy in these diseases is still uncertain.[169][170][171]Cyclophosphamideis sometimes used to treatlupus nephritis,a common symptom ofsystemic lupus erythematosus.[172]Dexamethasonealong with eitherbortezomibormelphalanis commonly used as a treatment forAL amyloidosis.Recently, bortezomid in combination withcyclophosphamideand dexamethasone has also shown promise as a treatment for AL amyloidosis. Other drugs used to treatmyelomasuch aslenalidomidehave shown promise in treating AL amyloidosis.[173]
Chemotherapy drugs are also used inconditioning regimensprior to bone marrow transplant (hematopoietic stem cell transplant). Conditioning regimens are used to suppress the recipient's immune system in order to allow a transplant to engraft. Cyclophosphamide is a common cytotoxic drug used in this manner and is often used in conjunction withtotal body irradiation.Chemotherapeutic drugs may be used at high doses to permanently remove the recipient's bone marrow cells (myeloablative conditioning) or at lower doses that will prevent permanent bone marrow loss (non-myeloablative and reduced intensity conditioning).[174]When used in non-cancer setting, the treatment is still called "chemotherapy", and is often done in the same treatment centers used for people with cancer.
Occupational exposure and safe handling
edit‹ThetemplateManualis beingconsidered for merging.›
This sectionis written likea manual or guide.(June 2023) |
In the 1970s, antineoplastic (chemotherapy) drugs were identified as hazardous, and theAmerican Society of Health-System Pharmacists(ASHP) has since then introduced the concept ofhazardous drugsafter publishing a recommendation in 1983 regarding handling hazardous drugs. The adaptation of federal regulations came when the U.S.Occupational Safety and Health Administration(OSHA) first released its guidelines in 1986 and then updated them in 1996, 1999, and, most recently, 2006.[175]
TheNational Institute for Occupational Safety and Health(NIOSH) has been conducting an assessment in the workplace since then regarding these drugs. Occupational exposure to antineoplastic drugs has been linked to multiple health effects, including infertility and possible carcinogenic effects. A few cases have been reported by the NIOSH alert report, such as one in which a female pharmacist was diagnosed with papillary transitional cell carcinoma. Twelve years before the pharmacist was diagnosed with the condition, she had worked for 20 months in a hospital where she was responsible for preparing multiple antineoplastic drugs.[176]The pharmacist did not have any other risk factor for cancer, and therefore, her cancer was attributed to the exposure to the antineoplastic drugs, although a cause-and-effect relationship has not been established in the literature. Another case happened when a malfunction in biosafety cabinetry is believed to have exposed nursing personnel to antineoplastic drugs. Investigations revealed evidence of genotoxic biomarkers two and nine months after that exposure.
Routes of exposure
editAntineoplastic drugs are usually given throughintravenous,intramuscular,intrathecal,orsubcutaneousadministration. In most cases, before the medication is administered to the patient, it needs to be prepared and handled by several workers. Any worker who is involved in handling, preparing, or administering the drugs, or with cleaning objects that have come into contact with antineoplastic drugs, is potentially exposed to hazardous drugs.[177]Health care workers are exposed to drugs in different circumstances, such as when pharmacists and pharmacy technicians prepare and handle antineoplastic drugs and when nurses and physicians administer the drugs to patients. Additionally, those who are responsible for disposing antineoplastic drugs in health care facilities are also at risk of exposure.[178]
Dermal exposure is thought to be the main route of exposure due to the fact that significant amounts of the antineoplastic agents have been found in the gloves worn by healthcare workers who prepare, handle, and administer the agents. Another noteworthy route of exposure is inhalation of the drugs' vapors. Multiple studies have investigated inhalation as a route of exposure, and although air sampling has not shown any dangerous levels, it is still a potential route of exposure. Ingestion by hand to mouth is a route of exposure that is less likely compared to others because of the enforced hygienic standard in the health institutions. However, it is still a potential route, especially in the workplace, outside of a health institute. One can also be exposed to these hazardous drugs through injection byneedle sticks.Research conducted in this area has established that occupational exposure occurs by examining evidence in multiple urine samples from health care workers.[179]
Hazards
editHazardous drugs expose health care workers to serious health risks. Many studies show that antineoplastic drugs could have many side effects on the reproductive system, such as fetal loss, congenital malformation, and infertility. Health care workers who are exposed to antineoplastic drugs on many occasions have adverse reproductive outcomes such as spontaneous abortions, stillbirths, and congenital malformations. Moreover, studies have shown that exposure to these drugs leads to menstrual cycle irregularities. Antineoplastic drugs may also increase the risk of learning disabilities among children of health care workers who are exposed to these hazardous substances.[180]
Moreover, these drugs havecarcinogeniceffects. In the past five decades, multiple studies have shown the carcinogenic effects of exposure to antineoplastic drugs. Similarly, there have been research studies that linked alkylating agents with humans developing leukemias. Studies have reported elevated risk of breast cancer, nonmelanoma skin cancer, and cancer of the rectum among nurses who are exposed to these drugs. Other investigations revealed that there is a potentialgenotoxiceffect from anti-neoplastic drugs to workers in health care settings.[176]
Safe handling in health care settings
editAs of 2018, there were nooccupational exposure limitsset for antineoplastic drugs, i.e., OSHA or theAmerican Conference of Governmental Industrial Hygienists(ACGIH) have not set workplace safety guidelines.[181]
Preparation
editNIOSH recommends using aventilated cabinetthat is designed to decrease worker exposure. Additionally, it recommends training of all staff, the use of cabinets, implementing an initial evaluation of the technique of the safety program, and wearing protective gloves and gowns when opening drug packaging, handling vials, or labeling. When wearingpersonal protective equipment,one should inspect gloves for physical defects before use and always wear double gloves and protective gowns. Health care workers are also required to wash their hands with water and soap before and after working with antineoplastic drugs, change gloves every 30 minutes or whenever punctured, and discard them immediately in a chemotherapy waste container.[182]
The gowns used should be disposable gowns made of polyethylene-coated polypropylene. When wearing gowns, individuals should make sure that the gowns are closed and have long sleeves. When preparation is done, the final product should be completely sealed in a plastic bag.[183]
The health care worker should also wipe all waste containers inside the ventilated cabinet before removing them from the cabinet. Finally, workers should remove all protective wear and put them in a bag for their disposal inside the ventilated cabinet.[178]
Administration
editDrugs should only be administered using protective medical devices such as needle lists and closed systems and techniques such as priming of IV tubing by pharmacy personnel inside a ventilated cabinet. Workers should always wear personal protective equipment such as double gloves, goggles, and protective gowns when opening the outer bag and assembling the delivery system to deliver the drug to the patient, and when disposing of all material used in the administration of the drugs.[181]
Hospital workers should never remove tubing from an IV bag that contains an antineoplastic drug, and when disconnecting the tubing in the system, they should make sure the tubing has been thoroughly flushed. After removing the IV bag, the workers should place it together with other disposable items directly in the yellow chemotherapy waste container with the lid closed. Protective equipment should be removed and put into a disposable chemotherapy waste container. After this has been done, one should double bag the chemotherapy waste before or after removing one's inner gloves. Moreover, one must always wash one's hands with soap and water before leaving the drug administration site.[184]
Employee training
editAll employees whose jobs in health care facilities expose them to hazardous drugs must receive training. Training should include shipping and receiving personnel, housekeepers, pharmacists, assistants, and all individuals involved in the transportation and storage of antineoplastic drugs. These individuals should receive information and training to inform them of the hazards of the drugs present in their areas of work. They should be informed and trained on operations and procedures in their work areas where they can encounter hazards, different methods used to detect the presence of hazardous drugs and how the hazards are released, and the physical and health hazards of the drugs, including their reproductive and carcinogenic hazard potential. Additionally, they should be informed and trained on the measures they should take to avoid and protect themselves from these hazards. This information ought to be provided when health care workers come into contact with the drugs, that is, perform the initial assignment in a work area with hazardous drugs. Moreover, training should also be provided when new hazards emerge as well as when new drugs, procedures, or equipment are introduced.[181]
Housekeeping and waste disposal
editWhen performing cleaning and decontaminating the work area where antineoplastic drugs are used, one should make sure that there is sufficient ventilation to prevent the buildup of airborne drug concentrations. When cleaning the work surface, hospital workers should use deactivation and cleaning agents before and after each activity as well as at the end of their shifts. Cleaning should always be done using double protective gloves and disposable gowns. After employees finish up cleaning, they should dispose of the items used in the activity in a yellow chemotherapy waste container while still wearing protective gloves. After removing the gloves, they should thoroughly wash their hands with soap and water. Anything that comes into contact or has a trace of the antineoplastic drugs, such as needles, empty vials, syringes, gowns, and gloves, should be put in the chemotherapy waste container.[185]
Spill control
editA written policy needs to be in place in case of a spill of antineoplastic products. The policy should address the possibility of various sizes of spills as well as the procedure and personal protective equipment required for each size. A trained worker should handle a large spill and always dispose of all cleanup materials in the chemical waste container according to EPA regulations, not in a yellow chemotherapy waste container.[186]
Occupational monitoring
editAmedical surveillanceprogram must be established. In case of exposure, occupational health professionals need to ask for a detailed history and do a thorough physical exam. They should test the urine of the potentially exposed worker by doing aurine dipstickor microscopic examination, mainly looking for blood, as several antineoplastic drugs are known to cause bladder damage.[176]
Urinary mutagenicity is a marker of exposure to antineoplastic drugs that was first used by Falck and colleagues in 1979 and uses bacterial mutagenicity assays. Apart from being nonspecific, the test can be influenced by extraneous factors such as dietary intake and smoking and is, therefore, used sparingly. However, the test played a significant role in changing the use of horizontal flow cabinets to vertical flow biological safety cabinets during the preparation of antineoplastic drugs because the former exposed health care workers to high levels of drugs. This changed the handling of drugs and effectively reduced workers' exposure to antineoplastic drugs.[176]
Biomarkers of exposure to antineoplastic drugs commonly include urinaryplatinum,methotrexate,urinarycyclophosphamideandifosfamide,and urinary metabolite of5-fluorouracil.In addition to this, there are other drugs used to measure the drugs directly in the urine, although they are rarely used. A measurement of these drugs directly in one's urine is a sign of high exposure levels and that an uptake of the drugs is happening either through inhalation or dermally.[176]
Available agents
editThere is an extensivelist of antineoplastic agents.Several classification schemes have been used to subdivide the medicines used for cancer into several different types.[187][188]
History
editThe first use ofsmall-molecule drugsto treat cancer was in the early 20th century, although the specific chemicals first used were not originally intended for that purpose.Mustard gaswas used as achemical warfareagent duringWorld War Iand was discovered to be a potent suppressor ofhematopoiesis(blood production).[189]A similar family of compounds known asnitrogen mustardswere studied further duringWorld War IIat theYale School of Medicine.[190]It was reasoned that an agent that damaged the rapidly growing white blood cells might have a similar effect on cancer.[190]Therefore, in December 1942, several people with advancedlymphomas(cancers of the lymphatic system and lymph nodes) were given the drug by vein, rather than by breathing the irritating gas.[190]Their improvement, although temporary, was remarkable.[191]Concurrently, during a military operation in World War II, following a Germanair raidon the Italian harbour ofBari,several hundred people were accidentally exposed to mustard gas, which had been transported there by theAllied forcesto prepare for possible retaliation in the event of German use of chemical warfare. The survivors were later found to have very low white blood cell counts.[192]After WWII was over and the reports declassified, the experiences converged and led researchers to look for other substances that might have similar effects against cancer. The first chemotherapy drug to be developed from this line of research wasmustine.Since then, many other drugs have been developed to treat cancer, and drug development has exploded into a multibillion-dollar industry, although the principles and limitations of chemotherapy discovered by the early researchers still apply.[193]
The termchemotherapy
editThe wordchemotherapywithout a modifier usually refers to cancer treatment, but its historical meaning was broader. The term was coined in the early 1900s byPaul Ehrlichas meaning any use of chemicals to treat any disease (chemo-+-therapy), such as the use ofantibiotics(antibacterial chemotherapy).[194]Ehrlich was not optimistic that effective chemotherapy drugs would be found for the treatment of cancer.[194]The first modern chemotherapeutic agent wasarsphenamine,an arsenic compound discovered in 1907 and used to treatsyphilis.[195]This was later followed bysulfonamides(sulfa drugs) andpenicillin.In today'susage,thesense"any treatment of disease with drugs" is often expressed with the wordpharmacotherapy.
Research
editTargeted delivery vehicles
editSpecially targeted delivery vehicles aim to increase effective levels of chemotherapy for tumor cells while reducing effective levels for other cells. This should result in an increased tumor kill or reduced toxicity or both.[196]
Antibody-drug conjugates
editAntibody-drug conjugates(ADCs) comprise anantibody,drug and a linker between them. The antibody will be targeted at a preferentially expressed protein in the tumour cells (known as atumor antigen) or on cells that the tumor can utilise, such as blood vesselendothelial cells.They bind to the tumor antigen and are internalised, where the linker releases the drug into the cell. These specially targeted delivery vehicles vary in their stability, selectivity, and choice of target, but, in essence, they all aim to increase the maximum effective dose that can be delivered to the tumor cells.[197]Reduced systemic toxicity means that they can also be used in people who are sicker and that they can carry new chemotherapeutic agents that would have been far too toxic to deliver via traditional systemic approaches.[198]
The first approved drug of this type wasgemtuzumab ozogamicin(Mylotarg), released byWyeth(nowPfizer). The drug was approved to treatacute myeloid leukemia.[199]Two other drugs,trastuzumab emtansineandbrentuximab vedotin,are both in late clinical trials, and the latter has been granted accelerated approval for the treatment ofrefractoryHodgkin's lymphomaand systemicanaplastic large cell lymphoma.[197]
Nanoparticles
editNanoparticlesare 1–1000nanometer(nm) sized particles that can promote tumor selectivity and aid in delivering low-solubilitydrugs. Nanoparticles can be targeted passively or actively. Passive targeting exploits the difference between tumor blood vessels and normal blood vessels. Blood vessels in tumors are "leaky" because they have gaps from 200 to 2000 nm, which allow nanoparticles to escape into the tumor. Active targeting uses biological molecules (antibodies,proteins,DNAandreceptor ligands) to preferentially target the nanoparticles to the tumor cells. There are many types of nanoparticle delivery systems, such assilica,polymers,liposomes[200]and magnetic particles. Nanoparticles made of magnetic material can also be used to concentrate agents at tumor sites using an externally applied magnetic field.[196]They have emerged as a useful vehicle inmagnetic drug deliveryfor poorly soluble agents such aspaclitaxel.[201]
Electrochemotherapy
editElectrochemotherapy is the combined treatment in which injection of a chemotherapeutic drug is followed by application of high-voltage electric pulses locally to the tumor. The treatment enables the chemotherapeutic drugs, which otherwise cannot or hardly go through the membrane of cells (such as bleomycin and cisplatin), to enter the cancer cells. Hence, greater effectiveness of antitumor treatment is achieved.[202]
Clinical electrochemotherapy has been successfully used for treatment of cutaneous and subcutaneous tumors irrespective of their histological origin.[203][204]The method has been reported as safe, simple and highly effective in all reports on clinical use of electrochemotherapy. According to the ESOPE project (European Standard Operating Procedures of Electrochemotherapy), the Standard Operating Procedures (SOP) for electrochemotherapy were prepared, based on the experience of the leading European cancer centres on electrochemotherapy.[205][206]Recently, new electrochemotherapy modalities have been developed for treatment of internal tumors using surgical procedures, endoscopic routes or percutaneous approaches to gain access to the treatment area.[207][208]
Hyperthermia therapy
editHyperthermia therapyis heat treatment for cancer that can be a powerful tool when used in combination with chemotherapy (thermochemotherapy) or radiation for the control of a variety of cancers. The heat can be applied locally to the tumor site, which will dilate blood vessels to the tumor, allowing more chemotherapeutic medication to enter the tumor. Additionally, the tumor cell membrane will become more porous, further allowing more of the chemotherapeutic medicine to enter the tumor cell.
Hyperthermia has also been shown to help prevent or reverse "chemo-resistance." Chemotherapy resistance sometimes develops over time as the tumors adapt and can overcome the toxicity of the chemo medication. "Overcoming chemoresistance has been extensively studied within the past, especially using CDDP-resistant cells. In regard to the potential benefit that drug-resistant cells can be recruited for effective therapy by combining chemotherapy with hyperthermia, it was important to show that chemoresistance against several anticancer drugs (e.g. mitomycin C, anthracyclines, BCNU, melphalan) including CDDP could be reversed at least partially by the addition of heat.[209]
Other animals
editChemotherapy is used in veterinary medicine similar to how it is used in human medicine.[210]
See also
edit- Anti-Cancer Drugs(journal)
- Antimicrobial chemotherapy
- Cancer and nausea
- Cancer-related fatigue
- Chemo brain
- Chemotherapy regimens
- Cytostasis
- Experimental cancer treatments
- Safe Handling of Hazardous Drugs
- Drug delivery
- Hyperthermia therapy
- Immunotherapy
- National Comprehensive Cancer Network
- Ototoxicity
- Radiation induced cognitive decline
- Radiotherapy
- Virotherapy
References
edit- ^abAlfarouk KO, Stock CM, Taylor S, Walsh M, Muddathir AK, Verduzco D, et al. (15 July 2015)."Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp".Cancer Cell International.15(1): 71.doi:10.1186/s12935-015-0221-1.PMC4502609.PMID26180516.
- ^Johnstone RW, Ruefli AA, Lowe SW (January 2002)."Apoptosis: a link between cancer genetics and chemotherapy".Cell.108(2): 153–64.doi:10.1016/S0092-8674(02)00625-6.PMID11832206.S2CID7429296.
- ^Rajman L, Chwalek K, Sinclair DA (2018)."Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence".Cell Metabolism.27(3): 529–547.doi:10.1016/j.cmet.2018.02.011.PMC6342515.PMID29514064.
- ^abcdefCorrie PG, Pippa G. (2008). "Cytotoxic chemotherapy: clinical aspects".Medicine.36(1): 24–28.doi:10.1016/j.mpmed.2007.10.012.
- ^abcWagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, Ho J, Unverzagt S (August 2017)."Chemotherapy for advanced gastric cancer".The Cochrane Database of Systematic Reviews.2017(8): CD004064.doi:10.1002/14651858.cd004064.pub4.PMC6483552.PMID28850174.
- ^abcdefghijkRachel Airley (2009).Cancer chemotherapy.Wiley-Blackwell.ISBN978-0-470-09254-5.
- ^abcdefWood M, Brighton D (2005).The Royal Marsden Hospital handbook of cancer chemotherapy: a guide for the multidisciplinary team.St. Louis, Mo: Elsevier Churchill Livingstone.ISBN978-0-443-07101-0.
- ^Perry, Michael J. (2008).The Chemotherapy source book.Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins.ISBN978-0-7817-7328-7.
- ^Epstein RJ (August 2005)."Maintenance therapy to suppress micrometastasis: the new challenge for adjuvant cancer treatment".Clinical Cancer Research.11(15): 5337–41.doi:10.1158/1078-0432.CCR-05-0437.PMID16061845.
- ^Skeel RT (2003).Handbook of Cancer Chemotherapy (paperback)(6th ed.). Lippincott Williams & Wilkins.ISBN978-0-7817-3629-9.
- ^Chabner B, Longo DL (2005).Cancer Chemotherapy and Biotherapy: Principles and Practice(4th ed.). Philadelphia: Lippincott Willians & Wilkins.ISBN978-0-7817-5628-0.
- ^Nastoupil LJ, Rose AC, Flowers CR (May 2012). "Diffuse large B-cell lymphoma: current treatment approaches".Oncology.26(5): 488–95.PMID22730604.
- ^Freedman A (October 2012)."Follicular lymphoma: 2012 update on diagnosis and management".American Journal of Hematology.87(10): 988–95.doi:10.1002/ajh.23313.PMID23001911.S2CID35447562.
- ^Rampling R, James A, Papanastassiou V (June 2004)."The present and future management of malignant brain tumours: surgery, radiotherapy, chemotherapy".Journal of Neurology, Neurosurgery, and Psychiatry.75(Suppl 2): ii24-30.doi:10.1136/jnnp.2004.040535.PMC1765659.PMID15146036.
- ^Madan V, Lear JT, Szeimies RM (February 2010)."Non-melanoma skin cancer".Lancet.375(9715): 673–85.doi:10.1016/S0140-6736(09)61196-X.PMC3339125.PMID20171403.
- ^Du Bois D, Du Bois EF (1989). "A formula to estimate the approximate surface area if height and weight be known. 1916".Nutrition.5(5): 303–11, discussion 312–3.PMID2520314.
- ^abcFelici A, Verweij J, Sparreboom A (September 2002). "Dosing strategies for anticancer drugs: the good, the bad and body-surface area".European Journal of Cancer.38(13): 1677–84.doi:10.1016/s0959-8049(02)00151-x.PMID12175683.
- ^abcKaestner SA, Sewell GJ (February 2007). "Chemotherapy dosing part I: scientific basis for current practice and use of body surface area".Clinical Oncology.19(1): 23–37.doi:10.1016/j.clon.2006.10.010.hdl:10026.1/3714.PMID17305252.
- ^Pinkel D (August 1958). "The use of body surface area as a criterion of drug dosage in cancer chemotherapy".Cancer Research.18(7): 853–6.PMID13573353.
- ^abGurney H (April 2002)."How to calculate the dose of chemotherapy".British Journal of Cancer.86(8): 1297–302.doi:10.1038/sj.bjc.6600139.PMC2375356.PMID11953888.
- ^abBeumer JH, Chu E, Salamone SJ (November 2012)."Body-surface area-based chemotherapy dosing: appropriate in the 21st century?".Journal of Clinical Oncology.30(31): 3896–7.doi:10.1200/JCO.2012.44.2863.PMID22965963.
- ^abBaker SD, Verweij J, Rowinsky EK, Donehower RC, Schellens JH, Grochow LB, Sparreboom A (December 2002)."Role of body surface area in dosing of investigational anticancer agents in adults, 1991-2001".Journal of the National Cancer Institute.94(24): 1883–8.doi:10.1093/jnci/94.24.1883.PMID12488482.
- ^abcdefghijGamelin E, Delva R, Jacob J, Merrouche Y, Raoul JL, Pezet D, Dorval E, Piot G, Morel A, Boisdron-Celle M (May 2008)."Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer".Journal of Clinical Oncology.26(13): 2099–105.doi:10.1200/jco.2007.13.3934.PMID18445839.S2CID9557055.
- ^Saam J, Critchfield GC, Hamilton SA, Roa BB, Wenstrup RJ, Kaldate RR (September 2011). "Body surface area-based dosing of 5-fluoruracil results in extensive interindividual variability in 5-fluorouracil exposure in colorectal cancer patients on FOLFOX regimens".Clinical Colorectal Cancer.10(3): 203–6.doi:10.1016/j.clcc.2011.03.015.PMID21855044.
- ^abcdefgCapitain O, Asevoaia A, Boisdron-Celle M, Poirier AL, Morel A, Gamelin E (December 2012). "Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: a phase II, proof-of-concept study".Clinical Colorectal Cancer.11(4): 263–7.doi:10.1016/j.clcc.2012.05.004.PMID22683364.
- ^Kaldate RR, Haregewoin A, Grier CE, Hamilton SA, McLeod HL (2012)."Modeling the 5-fluorouracil area under the curve versus dose relationship to develop a pharmacokinetic dosing algorithm for colorectal cancer patients receiving FOLFOX6".The Oncologist.17(3): 296–302.doi:10.1634/theoncologist.2011-0357.PMC3316912.PMID22382460.
- ^abcHunter RJ, Navo MA, Thaker PH, Bodurka DC, Wolf JK, Smith JA (February 2009). "Dosing chemotherapy in obese patients: actual versus assigned body surface area (BSA)".Cancer Treatment Reviews.35(1): 69–78.doi:10.1016/j.ctrv.2008.07.005.PMID18922643.
- ^Canal, P.; Chatelut, E.; Guichard, S. (1998)."Practical treatment guide for dose individualisation in cancer chemotherapy".Drugs.56(6): 1019–1038.doi:10.2165/00003495-199856060-00006.ISSN0012-6667.PMID9878990.S2CID36211632.
- ^Hanna, Louise; Crosby, Tom; Fergus Macbeth, eds. (2008).Practical clinical oncology.Cambridge, UK: Cambridge University Press.ISBN978-0-521-61816-8.
- ^Buffery PJ, Allen KM, Chin PK, Moore GA, Barclay ML, Begg EJ (February 2014). "Thirteen years' experience of pharmacokinetic monitoring and dosing of busulfan: can the strategy be improved?".Therapeutic Drug Monitoring.36(1): 86–92.doi:10.1097/FTD.0b013e31829dc940.PMID24299921.S2CID28646787.
- ^Bartelink IH, Bredius RG, Belitser SV, Suttorp MM, Bierings M, Knibbe CA, et al. (2014). "Association Between Busulfan Exposure and Outcome in Children Receiving Intravenous Busulfan Before Hematopoietic Stem Cell Transplantation".Ther Drug Monit.36(1): 93–99.doi:10.1097/FTD.0b013e3182a04fc7.PMID24061446.S2CID21072472.
- ^"ARK Methotrexate Assay".Ark Diagnostics. Archived fromthe originalon 28 April 2014.Retrieved28 April2014.
- ^"Customizing Chemotherapy for Better Cancer Care".My Care Diagnostics. Archived fromthe originalon 28 April 2014.Retrieved25 November2018.
- ^"A Brief History of BSA Dosing".My Care Diagnostics.
- ^Asher V, Lee J, Bali A (September 2012). "Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer".Medical Oncology.29(3): 2005–9.doi:10.1007/s12032-011-0019-5.PMID21735143.S2CID19558612.
- ^abSiddik ZH (2005). "Mechanisms of Action of Cancer Chemotherapeutic Agents: DNA-Interactive Alkylating Agents and Antitumour Platinum-Based Drugs".The Cancer Handbook.John Wiley & Sons, Ltd.doi:10.1002/0470025077.chap84b.ISBN978-0470025062.
- ^abcdefLind M.J., M.J. (2008). "Principles of cytotoxic chemotherapy".Medicine.36(1): 19–23.doi:10.1016/j.mpmed.2007.10.003.
- ^abcDamia G, D'Incalci M (September 1998)."Mechanisms of resistance to alkylating agents".Cytotechnology.27(1–3): 165–73.doi:10.1023/A:1008060720608.PMC3449574.PMID19002790.
- ^abcdMalhotra V, Perry MC (2003)."Classical chemotherapy: mechanisms, toxicities and the therapeutic window".Cancer Biology & Therapy.2(4 Suppl 1): S2-4.doi:10.4161/cbt.199.PMID14508075.
- ^Giorgi-Renault S, Renault J, Baron M, Gebel-Servolles P, Delic J, Cros S, Paoletti C (1988)."Heterocyclic quinones XIII. Dimerization in the series of 5,8-quinazolinediones: Synthesis and anti tumor effects of bis(4-amino-5,8-quinazolinediones)".Chem. Pharm. Bull.36(10): 3933–3947.doi:10.1248/cpb.36.3933.PMID3245973.
- ^Takimoto CH, Calvo E (2008)."Principles of Oncologic Pharmacotherapy".In Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (eds.).Cancer Management: A Multidisciplinary Approach(11th ed.). Archived fromthe originalon 15 May 2009.Retrieved18 June2009.
- ^abcParker WB (July 2009)."Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer".Chemical Reviews.109(7): 2880–93.doi:10.1021/cr900028p.PMC2827868.PMID19476376.
- ^Adjei AA (June 2004). "Pemetrexed (ALIMTA), a novel multitargeted antineoplastic agent".Clinical Cancer Research.10(12 Pt 2): 4276s–4280s.doi:10.1158/1078-0432.CCR-040010.PMID15217974.S2CID31467685.
- ^Wagstaff AJ, Ibbotson T, Goa KL (2003). "Capecitabine: a review of its pharmacology and therapeutic efficacy in the management of advanced breast cancer".Drugs.63(2): 217–36.doi:10.2165/00003495-200363020-00009.PMID12515569.
- ^Rowinsky EK, Donehower RC (October 1991). "The clinical pharmacology and use of antimicrotubule agents in cancer chemotherapeutics".Pharmacology & Therapeutics.52(1): 35–84.doi:10.1016/0163-7258(91)90086-2.PMID1687171.
- ^Vitale, Ilio; Galluzzi, Lorenzo; Castedo, Maria; Kroemer, Guido (June 2011)."Mitotic catastrophe: a mechanism for avoiding genomic instability".Nature Reviews Molecular Cell Biology.12(6): 385–392.doi:10.1038/nrm3115.ISSN1471-0072.PMID21527953.S2CID22483746.
- ^abcYue QX, Liu X, Guo DA (August 2010)."Microtubule-binding natural products for cancer therapy".Planta Medica.76(11): 1037–43.doi:10.1055/s-0030-1250073.PMID20577942.
- ^Hirata K, Miyamoto K, Miura Y (1994)."Catharanthus roseusL. (Periwinkle): Production of Vindoline and Catharanthine in Multiple Shoot Cultures ".In Bajaj YP (ed.).Biotechnology in Agriculture and Forestry 26.Medicinal and Aromatic Plants. Vol. VI.Springer-Verlag.pp.46–55.ISBN9783540563914.
- ^van Der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R (March 2004). "The Catharanthus alkaloids: pharmacognosy and biotechnology".Current Medicinal Chemistry.11(5): 607–28.doi:10.2174/0929867043455846.PMID15032608.
- ^Gansäuer A, Justicia J, Fan CA, Worgull D, Piestert F (2007)."Reductive C—C bond formation after epoxide opening via electron transfer".InKrische MJ(ed.).Metal Catalyzed Reductive C—C Bond Formation: A Departure from Preformed Organometallic Reagents.Topics in Current Chemistry. Vol. 279.Springer Science & Business Media.pp. 25–52.doi:10.1007/128_2007_130.ISBN9783540728795.
- ^Cooper R, Deakin JJ (2016)."Africa's gift to the world".Botanical Miracles: Chemistry of Plants That Changed the World.CRC Press.pp. 46–51.ISBN9781498704304.
- ^abKeglevich P, Hazai L, Kalaus G, Szántay C (May 2012)."Modifications on the basic skeletons of vinblastine and vincristine".Molecules.17(5): 5893–914.doi:10.3390/molecules17055893.PMC6268133.PMID22609781.
- ^Raviña, Enrique (2011)."Vinca alkaloids".The evolution of drug discovery: From traditional medicines to modern drugs.John Wiley & Sons.pp. 157–159.ISBN9783527326693.
- ^Faller BA, Pandit TN (2011)."Safety and efficacy of vinorelbine in the treatment of non-small cell lung cancer".Clinical Medicine Insights: Oncology.5:131–44.doi:10.4137/CMO.S5074.PMC3117629.PMID21695100.
- ^Ngo QA, Roussi F, Cormier A, Thoret S, Knossow M, Guénard D, Guéritte F (January 2009). "Synthesis and biological evaluation of vinca alkaloids and phomopsin hybrids".Journal of Medicinal Chemistry.52(1): 134–42.doi:10.1021/jm801064y.PMID19072542.
- ^Croteau, Rodney; Ketchum, Raymond E. B.; Long, Robert M.; Kaspera, Rüdiger; Wildung, Mark R. (2006)."Taxol biosynthesis and molecular genetics".Phytochemistry Reviews.5(1): 75–97.Bibcode:2006PChRv...5...75C.doi:10.1007/s11101-005-3748-2.ISSN1568-7767.PMC2901146.PMID20622989.
- ^Damayanthi Y, Lown JW (June 1998). "Podophyllotoxins: current status and recent developments".Current Medicinal Chemistry.5(3): 205–52.doi:10.2174/0929867305666220314204426.PMID9562603.S2CID247493530.
- ^Liu YQ, Yang L, Tian X (2007). "Podophyllotoxin: current perspectives".Current Bioactive Compounds.3(1): 37–66.doi:10.1016/j.jallcom.2006.06.070.
- ^Lodish H, Berk A, Zipursky SL, et al. (2000).Molecular Cell Biology. 4th edition. The Role of Topoisomerases in DNA Replication.New York: W. H. Freeman.
- ^Goodsell DS (2002)."The molecular perspective: DNA topoisomerases".Stem Cells.20(5): 470–1.doi:10.1634/stemcells.20-5-470.PMID12351817.S2CID9257158.
- ^Nitiss JL (May 2009)."Targeting DNA topoisomerase II in cancer chemotherapy".Nature Reviews. Cancer.9(5): 338–50.doi:10.1038/nrc2607.PMC2748742.PMID19377506.
- ^Offermanns S, Rosenthal W (14 August 2008).Encyclopedia of Molecular Pharmacology.Springer Science & Business Media. p. 155.ISBN9783540389163.
- ^Offermanns S, Rosenthal W (14 August 2008).Encyclopedia of Molecular Pharmacology.Springer Science & Business Media. pp. 91ff.ISBN9783540389163.
- ^Koeller J, Eble M (1988). "Mitoxantrone: A novel anthracycline derivative".Clinical Pharmacy.7(8): 574–81.PMID3048848.
- ^Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (June 2004). "Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity".Pharmacological Reviews.56(2): 185–229.doi:10.1124/pr.56.2.6.PMID15169927.S2CID13138853.
- ^Sobell HM (August 1985)."Actinomycin and DNA transcription".Proceedings of the National Academy of Sciences of the United States of America.82(16): 5328–31.Bibcode:1985PNAS...82.5328S.doi:10.1073/pnas.82.16.5328.PMC390561.PMID2410919.
- ^Dorr RT (April 1992). "Bleomycin pharmacology: mechanism of action and resistance, and clinical pharmacokinetics".Seminars in Oncology.19(2 Suppl 5): 3–8.PMID1384141.
- ^Verweij J, Pinedo HM (October 1990). "Mitomycin C: mechanism of action, usefulness and limitations".Anti-Cancer Drugs.1(1): 5–13.doi:10.1097/00001813-199010000-00002.PMID2131038.
- ^Greer JA, Amoyal N, Nisotel L, Fishbein JN, MacDonald J, Stagl J, et al. (March 2016)."A Systematic Review of Adherence to Oral Antineoplastic Therapies".The Oncologist.21(3): 354–76.doi:10.1634/theoncologist.2015-0405.PMC4786357.PMID26921292.
- ^O'Grady, Naomi P.; Alexander, Mary; Burns, Lillian A.; Dellinger, E. Patchen; Garland, Jeffrey; Heard, Stephen O.; Lipsett, Pamela A.; Masur, Henry; Mermel, Leonard A.; Pearson, Michele L.; Raad, Issam I.; Randolph, Adrienne G.; Rupp, Mark E.; Saint, Sanjay (1 May 2011)."Guidelines for the Prevention of Intravascular Catheter-related Infections".Clinical Infectious Diseases.52(9): e162–e193.doi:10.1093/cid/cir257.ISSN1058-4838.PMC3106269.PMID21460264.
- ^Moreno-Ramirez D, de la Cruz-Merino L, Ferrandiz L, Villegas-Portero R, Nieto-Garcia A (2010)."Isolated limb perfusion for malignant melanoma: systematic review on effectiveness and safety".The Oncologist.15(4): 416–27.doi:10.1634/theoncologist.2009-0325.PMC3227960.PMID20348274.
- ^Verhoef C, de Wilt JH, ten Hagen TL, Eggermont AM (October 2008). "Isolated hepatic perfusion for the treatment of liver tumors: sunset or sunrise?".Surgical Oncology Clinics of North America.17(4): 877–94, xi.doi:10.1016/j.soc.2008.04.007.PMID18722924.
- ^Hendriks JM, Van Schil PE (1998). "Isolated lung perfusion for the treatment of pulmonary metastases".Surgical Oncology.7(1–2): 59–63.doi:10.1016/S0960-7404(98)00028-0.PMID10421507.
- ^Chitwood K, Etzkorn J, Cohen G (September 2013). "Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons".Dermatologic Surgery.39(9): 1306–16.doi:10.1111/dsu.12300.PMID23915332.S2CID597295.
- ^Estcourt, Lise J; Stanworth, Simon J; Hopewell, Sally; Doree, Carolyn; Trivella, Marialena; Massey, Edwin (29 April 2016)."Granulocyte transfusions for treating infections in people with neutropenia or neutrophil dysfunction".The Cochrane Database of Systematic Reviews.2016(4): CD005339.doi:10.1002/14651858.CD005339.pub2.ISSN1469-493X.PMC4930145.PMID27128488.
- ^Léger, Chantal S.; Nevill, Thomas J. (11 May 2004)."Hematopoietic stem cell transplantation: a primer for the primary care physician".Canadian Medical Association Journal.170(10): 1569–1577.doi:10.1503/cmaj.1011625.ISSN0820-3946.PMC400723.PMID15136552.
- ^Huang ES (2000).Internal medicine: handbook for clinicians, resident survival guide.Arlington, VA: Scrub Hill Press. p. 130.ISBN978-0-9645467-5-2.
- ^Elad S, Zadik Y, Hewson I, Hovan A, Correa ME, Logan R, Elting LS, Spijkervet FK, Brennan MT (August 2010). "A systematic review of viral infections associated with oral involvement in cancer patients: a spotlight on Herpesviridea".Supportive Care in Cancer.18(8): 993–1006.doi:10.1007/s00520-010-0900-3.PMID20544224.S2CID2969472.
- ^abcGafter-Gvili A, Fraser A, Paul M, Vidal L, Lawrie TA, van de Wetering MD, Kremer LC, Leibovici L (January 2012)."Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy".The Cochrane Database of Systematic Reviews.1(1): CD004386.doi:10.1002/14651858.CD004386.pub3.PMC4170789.PMID22258955.
- ^"Coriolus Versicolor".Cancer.org. 10 June 2008. Archived fromthe originalon 25 June 2010.Retrieved7 August2012.
- ^Commissioner, Office of the (12 February 2021)."FDA Approves Drug to Reduce Bone Marrow Suppression Caused by Chemotherapy".FDA.Retrieved9 March2021.
- ^Davila ML (January 2006). "Neutropenic enterocolitis".Current Opinion in Gastroenterology.22(1): 44–7.doi:10.1097/01.mog.0000198073.14169.3b.PMID16319675.S2CID25479771.
- ^Sinicrope, Frank A. (2003)."Typhlitis".Holland-Frei Cancer Medicine. 6th Edition.
- ^abKeidan RD, Fanning J, Gatenby RA, Weese JL (March 1989). "Recurrent typhlitis. A disease resulting from aggressive chemotherapy".Diseases of the Colon and Rectum.32(3): 206–9.doi:10.1007/BF02554529.PMID2920627.S2CID46090832.
- ^Gibson RJ, Keefe DM (September 2006). "Cancer chemotherapy-induced diarrhoea and constipation: mechanisms of damage and prevention strategies".Supportive Care in Cancer.14(9): 890–900.doi:10.1007/s00520-006-0040-y.PMID16604351.S2CID109778.
- ^Wei D, Heus P, van de Wetering FT, van Tienhoven G, Verleye L, Scholten RJ (August 2018)."Probiotics for the prevention or treatment of chemotherapy- or radiotherapy-related diarrhoea in people with cancer".The Cochrane Database of Systematic Reviews.2018(8): CD008831.doi:10.1002/14651858.cd008831.pub3.PMC6513393.PMID30168576.
- ^Rodrigues, Fabio G; Dasilva, Giovanna; Wexner, Steven D (7 January 2017)."Neutropenic enterocolitis".World Journal of Gastroenterology.23(1): 42–47.doi:10.3748/wjg.v23.i1.42.ISSN1007-9327.PMC5221285.PMID28104979.
- ^Groopman JE, Itri LM (October 1999)."Chemotherapy-induced anemia in adults: incidence and treatment".Journal of the National Cancer Institute.91(19): 1616–34.doi:10.1093/jnci/91.19.1616.PMID10511589.
- ^Henry DH (July 2006). "The role of intravenous iron in cancer-related anemia".Oncology.20(8 Suppl 6): 21–4.PMID16925107.
- ^Rodgers GM, Becker PS, Bennett CL, Cella D, Chanan-Khan A, Chesney C, Cleeland C, Coccia PF, Djulbegovic B, Garst JL, Gilreath JA, Kraut EH, Lin WC, Matulonis U, Millenson M, Reinke D, Rosenthal J, Sabbatini P, Schwartz RN, Stein RS, Vij R (July 2008). "Cancer- and chemotherapy-induced anemia".Journal of the National Comprehensive Cancer Network.6(6): 536–64.doi:10.6004/jnccn.2008.0042.PMID18597709.
- ^Vadhan-Raj S (January 2009). "Management of chemotherapy-induced thrombocytopenia: current status of thrombopoietic agents".Seminars in Hematology.46(1 Suppl 2): S26-32.doi:10.1053/j.seminhematol.2008.12.007.PMID19245931.
- ^Sekhon SS, Roy V (May 2006). "Thrombocytopenia in adults: A practical approach to evaluation and management".Southern Medical Journal.99(5): 491–8, quiz 499–500, 533.doi:10.1097/01.smj.0000209275.75045.d4.PMID16711312.S2CID16527763.
- ^Estcourt L, Stanworth S, Doree C, Hopewell S, Murphy MF, Tinmouth A, Heddle N, et al. (Cochrane Haematological Malignancies Group) (May 2012)."Prophylactic platelet transfusion for prevention of bleeding in patients with haematological disorders after chemotherapy and stem cell transplantation".The Cochrane Database of Systematic Reviews(5): CD004269.doi:10.1002/14651858.CD004269.pub3.PMID22592695.
- ^Estcourt LJ, Stanworth SJ, Doree C, Hopewell S, Trivella M, Murphy MF, et al. (Cochrane Haematological Malignancies Group) (November 2015)."Comparison of different platelet count thresholds to guide administration of prophylactic platelet transfusion for preventing bleeding in people with haematological disorders after myelosuppressive chemotherapy or stem cell transplantation".The Cochrane Database of Systematic Reviews.2015(11): CD010983.doi:10.1002/14651858.CD010983.pub2.PMC4717525.PMID26576687.
- ^Berger AM, Abernethy AP, Atkinson A, Barsevick AM, Breitbart WS, Cella D, Cimprich B, Cleeland C, Eisenberger MA, Escalante CP, Jacobsen PB, Kaldor P, Ligibel JA, Murphy BA, O'Connor T, Pirl WF, Rodler E, Rugo HS, Thomas J, Wagner LI (August 2010)."NCCN Clinical Practice Guidelines Cancer-related fatigue".Journal of the National Comprehensive Cancer Network.8(8): 904–31.doi:10.6004/jnccn.2010.0067.PMID20870636.S2CID70400559.
- ^Franklin DJ, Packel L (March 2006). "Cancer-related fatigue".Archives of Physical Medicine and Rehabilitation.87(3 Suppl 1): S91–3, quiz S94–5.doi:10.1016/j.apmr.2005.12.015.PMID16500197.
- ^Cramp F, Byron-Daniel J (November 2012). Cramp F (ed.)."Exercise for the management of cancer-related fatigue in adults".The Cochrane Database of Systematic Reviews.11(9): CD006145.doi:10.1002/14651858.CD006145.pub3.PMC8480137.PMID23152233.
- ^PDQ Supportive and Palliative Care Editorial Board (2002),"Nausea and Vomiting Related to Cancer Treatment (PDQ®): Health Professional Version",PDQ Cancer Information Summaries,Bethesda (MD): National Cancer Institute (US),PMID26389491,retrieved8 November2022
- ^Gill P, Grothey A, Loprinzi C (2006). "Nausea and Vomiting in the Cancer Patient".Oncology:1482–1496.doi:10.1007/0-387-31056-8_83.ISBN978-0-387-24291-0.
Nausea and vomiting are two of the most feared cancer treatment-related side effects for cancer patients and their families.
- ^Sanger, Gareth J.; Andrews, Paul L. R. (4 September 2018)."A History of Drug Discovery for Treatment of Nausea and Vomiting and the Implications for Future Research".Frontiers in Pharmacology.9:913.doi:10.3389/fphar.2018.00913.ISSN1663-9812.PMC6131675.PMID30233361.
- ^"Chemotherapy-Related Hair Loss (Alopecia) in Children - Health Encyclopedia - University of Rochester Medical Center".www.urmc.rochester.edu.Retrieved8 November2022.
- ^Chadha V, Shenoi SD (2003). "Hair loss in cancer chemotherapeutic patients".Indian Journal of Dermatology, Venereology and Leprology.69(2): 131–2.PMID17642856.
- ^Lemieux J (October 2012). "Reducing chemotherapy-induced alopecia with scalp cooling".Clinical Advances in Hematology & Oncology.10(10): 681–2.PMID23187775.
- ^Shapiro J, Price VH (April 1998). "Hair regrowth. Therapeutic agents".Dermatologic Clinics.16(2): 341–56.doi:10.1016/S0733-8635(05)70017-6.PMID9589208.
- ^Al-Mohanna H, Al-Khenaizan S (2010). "Permanent alopecia following cranial irradiation in a child".Journal of Cutaneous Medicine and Surgery.14(3): 141–3.doi:10.2310/7750.2010.09014.PMID20487675.S2CID43583651.
- ^Can G, Demir M, Erol O, Aydiner A (June 2013). "A comparison of men and women's experiences of chemotherapy-induced alopecia".European Journal of Oncology Nursing.17(3): 255–60.doi:10.1016/j.ejon.2012.06.003.PMID22901547.
- ^Trüeb RM (March 2009). "Chemotherapy-induced alopecia".Seminars in Cutaneous Medicine and Surgery.28(1): 11–4.doi:10.1016/j.sder.2008.12.001.PMID19341937.
- ^Chon SY, Champion RW, Geddes ER, Rashid RM (July 2012). "Chemotherapy-induced alopecia".Journal of the American Academy of Dermatology.67(1): e37-47.doi:10.1016/j.jaad.2011.02.026.PMID22178150.
- ^Secondary neoplasias following chemotherapy, radiotherapy and immunosuppression.Vol. 55. Basel: Karger. 2000.ISBN9783318006155.OCLC606559421.
- ^Hijiya N, Hudson MM, Lensing S, Zacher M, Onciu M, Behm FG, Razzouk BI, Ribeiro RC, Rubnitz JE, Sandlund JT, Rivera GK, Evans WE, Relling MV, Pui CH (March 2007)."Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia".JAMA.297(11): 1207–15.doi:10.1001/jama.297.11.1207.PMID17374815.
- ^abcdBrydøy M, Fosså SD, Dahl O, Bjøro T (2007). "Gonadal dysfunction and fertility problems in cancer survivors".Acta Oncologica.46(4): 480–9.doi:10.1080/02841860601166958.PMID17497315.S2CID20672988.
- ^abMorgan S, Anderson RA, Gourley C, Wallace WH, Spears N (2012)."How do chemotherapeutic agents damage the ovary?".Human Reproduction Update.18(5): 525–35.doi:10.1093/humupd/dms022.hdl:1842/9543.PMID22647504.
- ^Gurgan T, Salman C, Demirol A (October 2008). "Pregnancy and assisted reproduction techniques in men and women after cancer treatment".Placenta.29 Suppl B: 152–9.doi:10.1016/j.placenta.2008.07.007.PMID18790328.
- ^Courbiere B, Decanter C, Bringer-Deutsch S, Rives N, Mirallié S, Pech JC, De Ziegler D, Carré-Pigeon F, May-Panloup P, Sifer C, Amice V, Schweitzer T, Porcu-Buisson G, Poirot C (September 2013)."Emergency IVF for embryo freezing to preserve female fertility: a French multicentre cohort study".Human Reproduction.28(9): 2381–8.doi:10.1093/humrep/det268.PMID23832792.
- ^Roness H, Kalich-Philosoph L, Meirow D (2014)."Prevention of chemotherapy-induced ovarian damage: possible roles for hormonal and non-hormonal attenuating agents".Human Reproduction Update.20(5): 759–74.doi:10.1093/humupd/dmu019.PMID24833728.
- ^Tichelli A, Rovó A (August 2013). "Fertility issues following hematopoietic stem cell transplantation".Expert Review of Hematology.6(4): 375–88.doi:10.1586/17474086.2013.816507.PMID23991924.S2CID25139582.
In turn citing:Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ, Doney K, Storb R, Sullivan K, Witherspoon R, Appelbaum FR (April 1996)."Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation".Blood.87(7): 3045–52.doi:10.1182/blood.V87.7.3045.bloodjournal8773045.PMID8639928. - ^abcdefArnon J, Meirow D, Lewis-Roness H, Ornoy A (2001)."Genetic and teratogenic effects of cancer treatments on gametes and embryos".Human Reproduction Update.7(4): 394–403.doi:10.1093/humupd/7.4.394.PMID11476352.
- ^DOXORUBICIN HYDROCHLORIDE injection, solutiondrug label/data atDailyMedfromU.S. National Library of Medicine,National Institutes of Health.
- ^abdel Pino BM (23 February 2010)."Chemotherapy-induced Peripheral Neuropathy".NCI Cancer Bulletin.7(4): 6.
- ^Grisold W, Oberndorfer S, Windebank AJ (2012)."Chemotherapy and polyneuropathies"(PDF).European Association of Neurooncology Magazine.12(1).Archived(PDF)from the original on 12 August 2012.
- ^Beijers AJ, Jongen JL, Vreugdenhil G (January 2012)."Chemotherapy-induced neurotoxicity: the value of neuroprotective strategies".The Netherlands Journal of Medicine.70(1): 18–25.PMID22271810.
- ^abWindebank AJ, Grisold W (March 2008). "Chemotherapy-induced neuropathy".Journal of the Peripheral Nervous System.13(1): 27–46.doi:10.1111/j.1529-8027.2008.00156.x.PMID18346229.S2CID20411101.
- ^Savage L (July 2007)."Chemotherapy-induced pain puzzles scientists".Journal of the National Cancer Institute.99(14): 1070–1.doi:10.1093/jnci/djm072.PMID17623791.
- ^Tannock IF, Ahles TA, Ganz PA, Van Dam FS (June 2004)."Cognitive impairment associated with chemotherapy for cancer: report of a workshop".Journal of Clinical Oncology.22(11): 2233–9.doi:10.1200/JCO.2004.08.094.PMID15169812.
- ^Adeyinka, Adebayo; Bashir, Khalid (2022),"Tumor Lysis Syndrome",StatPearls,Treasure Island (FL): StatPearls Publishing,PMID30085527,retrieved8 November2022
- ^Shaikh AY, Shih JA (June 2012). "Chemotherapy-induced cardiotoxicity".Current Heart Failure Reports.9(2): 117–27.doi:10.1007/s11897-012-0083-y.PMID22382639.S2CID35723897.
- ^Thatishetty AV, Agresti N, O'Brien CB (November 2013). "Chemotherapy-induced hepatotoxicity".Clinics in Liver Disease.17(4): 671–86, ix–x.doi:10.1016/j.cld.2013.07.010.PMID24099024.
- ^King PD, Perry MC (2001)."Hepatotoxicity of chemotherapy"(PDF).The Oncologist.6(2): 162–76.doi:10.1634/theoncologist.6-2-162.PMID11306728.S2CID39518402.
- ^de Jonge MJ, Verweij J (February 2006). "Renal toxicities of chemotherapy".Seminars in Oncology.33(1): 68–73.doi:10.1053/j.seminoncol.2005.11.011.PMID16473645.
- ^Humphreys BD, Soiffer RJ, Magee CC (January 2005)."Renal failure associated with cancer and its treatment: an update".Journal of the American Society of Nephrology.16(1): 151–61.doi:10.1681/ASN.2004100843.PMID15574506.
- ^Brock PR, Knight KR, Freyer DR, Campbell KC, Steyger PS, Blakley BW, et al. (July 2012)."Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale".Journal of Clinical Oncology.30(19): 2408–17.doi:10.1200/JCO.2011.39.1110.PMC3675696.PMID22547603.
- ^Rybak LP, Mukherjea D, Jajoo S, Ramkumar V (November 2009)."Cisplatin ototoxicity and protection: clinical and experimental studies".The Tohoku Journal of Experimental Medicine.219(3): 177–86.doi:10.1620/tjem.219.177.PMC2927105.PMID19851045.
- ^van As JW, van den Berg H, van Dalen EC (January 2020)."Different infusion durations for preventing platinum-induced hearing loss in children with cancer".The Cochrane Database of Systematic Reviews.1(1): CD010885.doi:10.1002/14651858.CD010885.pub5.PMC6984653.PMID31961948.
- ^van As JW, van den Berg H, van Dalen EC (August 2016)."Platinum-induced hearing loss after treatment for childhood cancer".The Cochrane Database of Systematic Reviews.2019(8): CD010181.doi:10.1002/14651858.cd010181.pub2.PMC6466671.PMID27486906.
- ^van As JW, van den Berg H, van Dalen EC (May 2019)."Medical interventions for the prevention of platinum-induced hearing loss in children with cancer".The Cochrane Database of Systematic Reviews.2019(5): CD009219.doi:10.1002/14651858.cd009219.pub5.PMC6504134.PMID31063591.
- ^Shannon, Vickie R. (9 July 2019). "Cancer Treatment-Related Lung Injury".Oncologic Critical Care.pp. 531–556.doi:10.1007/978-3-319-74588-6_52.ISBN978-3-319-74587-9.PMC7123056.
- ^Kwakman, Johannes J.M.; Elshot, Yannick S.; Punt, Cornelis J.A.; Koopman, Miriam (13 May 2020)."Management of cytotoxic chemotherapy-induced hand-foot syndrome".Oncology Reviews.14(1): 442.doi:10.4081/oncol.2020.442.ISSN1970-5565.PMC7232019.PMID32431787.
- ^Ward EJ, Henry LM, Friend AJ, Wilkins S, Phillips RS (August 2015)."Nutritional support in children and young people with cancer undergoing chemotherapy".The Cochrane Database of Systematic Reviews.2015(8): CD003298.doi:10.1002/14651858.cd003298.pub3.PMC8752126.PMID26301790.
- ^Weeks JC, Catalano PJ, Cronin A, Finkelman MD, Mack JW, Keating NL, Schrag D (October 2012)."Patients' expectations about effects of chemotherapy for advanced cancer".The New England Journal of Medicine.367(17): 1616–25.doi:10.1056/NEJMoa1204410.PMC3613151.PMID23094723.
- ^Deeken JF, Löscher W (March 2007)."The blood-brain barrier and cancer: transporters, treatment, and Trojan horses".Clinical Cancer Research.13(6): 1663–74.doi:10.1158/1078-0432.CCR-06-2854.PMID17363519.
- ^Agarwala SS, Kirkwood JM (2000)."Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma".The Oncologist.5(2): 144–51.doi:10.1634/theoncologist.5-2-144.PMID10794805.S2CID25331035.
- ^Gerstner ER, Fine RL (June 2007). "Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm".Journal of Clinical Oncology.25(16): 2306–12.doi:10.1200/JCO.2006.10.0677.PMID17538177.
- ^Minchinton AI, Tannock IF (August 2006). "Drug penetration in solid tumours".Nature Reviews. Cancer.6(8): 583–92.doi:10.1038/nrc1893.PMID16862189.S2CID42818461.
- ^Goldman B (February 2003)."Multidrug resistance: can new drugs help chemotherapy score against cancer?".Journal of the National Cancer Institute.95(4): 255–7.doi:10.1093/jnci/95.4.255.PMID12591977.
- ^Crowley E, McDevitt CA, Callaghan R (2009).Multidrug Resistance in Cancer. Generating Inhibitors of P-Glycoprotein: Where to, Now?.Springer Protocols. pp. 405–432.
- ^Luqmani YA (2005)."Mechanisms of drug resistance in cancer chemotherapy".Medical Principles and Practice.14(Suppl 1): 35–48.doi:10.1159/000086183.PMID16103712.
- ^Moschovi M, Critselis E, Cen O, Adamaki M, Lambrou GI, Chrousos GP, Vlahopoulos S (2015). "Drugs acting on homeostasis: challenging cancer cell adaptation".Expert Review of Anticancer Therapy.15(12): 1405–17.doi:10.1586/14737140.2015.1095095.PMID26523494.S2CID28992964.
- ^Heavey S, Godwin P, Baird AM, Barr MP, Umezawa K, Cuffe S, et al. (October 2014)."Strategic targeting of the PI3K-NFκB axis in cisplatin-resistant NSCLC".Cancer Biology & Therapy.15(10): 1367–77.doi:10.4161/cbt.29841.PMC4130730.PMID25025901.
- ^Ryan SL, Beard S, Barr MP, Umezawa K, Heavey S, Godwin P, et al. (September 2019)."Targeting NF-κB-mediated inflammatory pathways in cisplatin-resistant NSCLC"(PDF).Lung Cancer.135:217–227.doi:10.1016/j.lungcan.2019.07.006.PMID31446998.S2CID199025494.
- ^Godwin P, Baird AM, Heavey S, Barr MP, O'Byrne KJ, Gately K (2013)."Targeting nuclear factor-kappa B to overcome resistance to chemotherapy".Frontiers in Oncology.3:120.doi:10.3389/fonc.2013.00120.PMC3655421.PMID23720710.
- ^Vanneman, Matthew; Dranoff, Glenn (22 March 2012)."Combining Immunotherapy and Targeted Therapies in Cancer Treatment".Nature Reviews. Cancer.12(4): 237–251.doi:10.1038/nrc3237.ISSN1474-175X.PMC3967236.PMID22437869.
- ^Gerber DE (February 2008). "Targeted therapies: a new generation of cancer treatments".American Family Physician.77(3): 311–9.PMID18297955.
- ^Allen TM (October 2002). "Ligand-targeted therapeutics in anticancer therapy".Nature Reviews. Cancer.2(10): 750–63.doi:10.1038/nrc903.PMID12360278.S2CID21014917.
- ^Chen HX, Cleck JN (August 2009)."Adverse effects of anticancer agents that target the VEGF pathway".Nature Reviews. Clinical Oncology.6(8): 465–77.doi:10.1038/nrclinonc.2009.94.PMID19581909.S2CID30482752.
- ^Zhang J, Yang PL, Gray NS (January 2009). "Targeting cancer with small molecule kinase inhibitors".Nature Reviews. Cancer.9(1): 28–39.doi:10.1038/nrc2559.PMID19104514.S2CID17934366.
- ^Hanahan D, Weinberg RA (January 2000)."The hallmarks of cancer".Cell.100(1): 57–70.doi:10.1016/S0092-8674(00)81683-9.PMID10647931.S2CID1478778.
- ^Hodgson S (January 2008)."Mechanisms of inherited cancer susceptibility".Journal of Zhejiang University Science B.9(1): 1–4.doi:10.1631/jzus.B073001.PMC2170461.PMID18196605.
- ^Perera FP (November 1997). "Environment and cancer: who are susceptible?".Science.278(5340): 1068–73.Bibcode:1997Sci...278.1068P.doi:10.1126/science.278.5340.1068.PMID9353182.
- ^Hoskins WJ, Perez CA, Young RC, Barakat RR, Markman M, Randall ME, eds. (2005).Principles and practice of gynecologic oncology(4th ed.). Baltimore, Md.: Lippincott Williams & Wilkins.ISBN978-0-7817-4689-2.
- ^Kehe K, Balszuweit F, Steinritz D, Thiermann H (September 2009). "Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering".Toxicology.263(1): 12–9.doi:10.1016/j.tox.2009.01.019.PMID19651324.
- ^Makin G, Hickman JA (July 2000). "Apoptosis and cancer chemotherapy".Cell and Tissue Research.301(1): 143–52.Bibcode:1994RSPTB.345..319H.doi:10.1007/s004419900160.PMID10928287.S2CID22909070.
- ^Fisher, R; Pusztai, L; Swanton, C (19 February 2013)."Cancer heterogeneity: implications for targeted therapeutics".British Journal of Cancer.108(3): 479–485.doi:10.1038/bjc.2012.581.ISSN0007-0920.PMC3593543.PMID23299535.
- ^Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G (January 2008)."Immunological aspects of cancer chemotherapy".Nature Reviews. Immunology.8(1): 59–73.doi:10.1038/nri2216.PMID18097448.S2CID205490545.
- ^Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G (February 2017). "Immunogenic cell death in cancer and infectious disease".Nature Reviews. Immunology.17(2): 97–111.doi:10.1038/nri.2016.107.PMID27748397.S2CID4045072.
- ^Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, Lin YJ, Wojtkiewicz G, Iwamoto Y, Mino-Kenudson M, Huynh TG, Hynes RO, Freeman GJ, Kroemer G, Zitvogel L, Weissleder R, Pittet MJ (February 2016)."Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy".Immunity.44(2): 343–54.doi:10.1016/j.immuni.2015.11.024.PMC4758865.PMID26872698.
- ^abBen-Ari ET (April 2004)."Dual purpose: some cancer therapies used to treat autoimmune diseases".Journal of the National Cancer Institute.96(8): 577–9.doi:10.1093/jnci/96.8.577.PMID15100330.
- ^abCutolo M, Sulli A, Pizzorni C, Seriolo B, Straub RH (August 2001)."Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis".Annals of the Rheumatic Diseases.60(8): 729–35.doi:10.1136/ard.60.8.729.PMC1753808.PMID11454634.
- ^Montaudié H, Sbidian E, Paul C, Maza A, Gallini A, Aractingi S, Aubin F, Bachelez H, Cribier B, Joly P, Jullien D, Le Maître M, Misery L, Richard MA, Ortonne JP (May 2011). "Methotrexate in psoriasis: a systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity".Journal of the European Academy of Dermatology and Venereology.25(Suppl 2): 12–8.doi:10.1111/j.1468-3083.2011.03991.x.PMID21388454.S2CID13015911.
- ^abChen J, Veras MM, Liu C, Lin J (February 2013). Chen J (ed.). "Methotrexate for ankylosing spondylitis".The Cochrane Database of Systematic Reviews.2(2): CD004524.doi:10.1002/14651858.CD004524.pub4.PMID23450553.
- ^abGray O, McDonnell GV, Forbes RB (2004). Gray O (ed.)."Methotrexate for multiple sclerosis".The Cochrane Database of Systematic Reviews.2010(2): CD003208.doi:10.1002/14651858.CD003208.pub2.PMC9006525.PMID15106195.
- ^abGray OM, McDonnell GV, Forbes RB (August 2006). "A systematic review of oral methotrexate for multiple sclerosis".Multiple Sclerosis.12(4): 507–10.doi:10.1191/1352458506ms1299oa.PMID16900766.S2CID46120577.
- ^Ntali S, Bertsias G, Boumpas DT (June 2011). "Cyclophosphamide and lupus nephritis: when, how, for how long?".Clinical Reviews in Allergy & Immunology.40(3): 181–91.doi:10.1007/s12016-009-8196-0.PMID20107927.S2CID11902371.
- ^"NCCN Guidelines: Systemic Light Chain Amyloidosis"(PDF).National Comprehensive Cancer Network. Archived fromthe original(PDF)on 25 June 2021.Retrieved25 February2015.
- ^Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, Apperley J, Slavin S, Pasquini M, Sandmaier BM, Barrett J, Blaise D, Lowski R, Horowitz M (December 2009)."Defining the intensity of conditioning regimens: working definitions".Biology of Blood and Marrow Transplantation.15(12): 1628–33.doi:10.1016/j.bbmt.2009.07.004.PMC2861656.PMID19896087.
- ^Sorsa M, Hämeilä M, Järviluoma E (September 2006). "Handling anticancer drugs: from hazard identification to risk management?".Annals of the New York Academy of Sciences.1076(1): 628–34.Bibcode:2006NYASA1076..628S.doi:10.1196/annals.1371.008.PMID17119240.S2CID7081365.
- ^abcdeThomas h. Connor, PhD (March 2014)."Chemotherapy: Biomarkers of Exposure, Effect, Reproductive Hazards, and Cancer".The Oncology Pharmacist.Archived fromthe originalon 25 June 2021.Retrieved22 November2018.
- ^"Chemotherapy Safety".Cancer.org.Archivedfrom the original on 10 August 2021.Retrieved10 August2021.
- ^ab"Personal protective equipment for health care workers who work with hazardous drugs" (Document). NIOSH. October 2008.doi:10.26616/NIOSHPUB2009106.
- ^Antineoplastic drugs: Occupational exposure and health risks.2006.ISBN978-90-393-4331-9.
- ^Connor, Thomas H.; Lawson, Christina C.; Polovich, Martha; McDiarmid, Melissa A. (2014)."Reproductive Health Risks Associated with Occupational Exposures to Antineoplastic Drugs in Health Care Settings: A Review of the Evidence".Journal of Occupational and Environmental Medicine.56(9): 901–910.doi:10.1097/JOM.0000000000000249.ISSN1076-2752.PMC4569003.PMID25153300.
- ^abc"preventing occupational exposure to antineoplastic and other hazardous drugs in health care settings"(PDF).Archived(PDF)from the original on 13 September 2004.
- ^"Hazardous Drugs Program Guides".lni.wa.gov.Archived fromthe originalon 27 October 2019.Retrieved22 November2018.
- ^Kilinc, F. Selcen (2015)."A Review of Isolation Gowns in Healthcare: Fabric and Gown Properties".Journal of Engineered Fibers and Fabrics.10(3): 180–190.doi:10.1177/155892501501000313.ISSN1558-9250.PMC4791533.PMID26989351.
- ^Reinhardt, Peter A. (29 November 2017).Infectious and Medical Waste Management.CRC Press.ISBN9781315894430.[page needed]
- ^"Safe management of wastes from health-care activities"(PDF).WHO.
- ^DeJoy DM, Smith TD, Woldu H, Dyal MA, Steege AL, Boiano JM (July 2017). "Effects of organizational safety practices and perceived safety climate on PPE usage, engineering controls, and adverse events involving liquid antineoplastic drugs among nurses".Journal of Occupational and Environmental Hygiene.14(7): 485–493.doi:10.1080/15459624.2017.1285496.PMID28326998.S2CID3879822.
- ^Ian Olver (19 October 2010).The MASCC Textbook of Cancer Supportive Care and Survivorship.Springer Science & Business Media. p. 351.ISBN978-1-4419-1225-1.
- ^Holland JF, Pollock RE (2010).Holland-Frei Cancer Medicine 8.PMPH-USA. p. 1758.ISBN978-1-60795-014-1.
- ^Krumbhaar EB (1919)."Role of the blood and the bone marrow in certain forms of gas poisoning".JAMA.72:39–41.doi:10.1001/jama.1919.26110010018009f.
- ^abcFenn JE, Udelsman R (March 2011). "First use of intravenous chemotherapy cancer treatment: rectifying the record".Journal of the American College of Surgeons.212(3): 413–7.doi:10.1016/j.jamcollsurg.2010.10.018.PMID21247779.
- ^Chabner BA, Roberts TG (January 2005). "Timeline: Chemotherapy and the war on cancer".Nature Reviews. Cancer.5(1): 65–72.doi:10.1038/nrc1529.PMID15630416.S2CID205467419.
- ^Faguet GB (2005).The War on Cancer.Springer. p.71.ISBN978-1-4020-3618-7.
- ^Joensuu H (March 2008). "Systemic chemotherapy for cancer: from weapon to treatment".The Lancet. Oncology.9(3): 304.doi:10.1016/S1470-2045(08)70075-5.PMID18308256.
- ^abDeVita VT, Chu E (November 2008)."A history of cancer chemotherapy".Cancer Research.68(21): 8643–53.doi:10.1158/0008-5472.CAN-07-6611.PMID18974103.
- ^Nichols HJ, Walker JE (March 1923)."Experimental Observations on the Prophylaxis and Treatment of Syphilis".The Journal of Experimental Medicine.37(4): 525–42.doi:10.1084/jem.37.4.525.PMC2128372.PMID19868743.
- ^abChidambaram M, Manavalan R, Kathiresan K (2011)."Nanotherapeutics to overcome conventional cancer chemotherapy limitations".Journal of Pharmacy & Pharmaceutical Sciences.14(1): 67–77.doi:10.18433/J30C7D.PMID21501554.
- ^abTeicher BA, Chari RV (October 2011)."Antibody conjugate therapeutics: challenges and potential".Clinical Cancer Research.17(20): 6389–97.doi:10.1158/1078-0432.CCR-11-1417.PMID22003066.
- ^Mokhtari, Reza Bayat; Homayouni, Tina S.; Baluch, Narges; Morgatskaya, Evgeniya; Kumar, Sushil; Das, Bikul; Yeger, Herman (30 March 2017)."Combination therapy in combating cancer".Oncotarget.8(23): 38022–38043.doi:10.18632/oncotarget.16723.ISSN1949-2553.PMC5514969.PMID28410237.
- ^Sievers EL, Linenberger M (November 2001). "Mylotarg: antibody-targeted chemotherapy comes of age".Current Opinion in Oncology.13(6): 522–7.doi:10.1097/00001622-200111000-00016.PMID11673694.S2CID27827980.
- ^Taléns-Visconti R, Díez-Sales O, de Julián-Ortiz JV, Nácher A (April 2022)."Nanoliposomes in Cancer Therapy: Marketed Products and Current Clinical Trials".International Journal of Molecular Sciences.23(8): 4249.doi:10.3390/ijms23084249.PMC9030431.PMID35457065.
- ^Vines T, Faunce T (May 2009). "Assessing the safety and cost-effectiveness of early nanodrugs".Journal of Law and Medicine.16(5): 822–45.PMID19554862.
- ^Larkin, John O.; Collins, Christopher G.; Aarons, Simon; Tangney, Mark; Whelan, Maria; O'Reily, Seamus; Breathnach, Oscar; Soden, Declan M.; O'Sullivan, Gerald C. (2007)."Electrochemotherapy".Annals of Surgery.245(3): 469–479.doi:10.1097/01.sla.0000250419.36053.33.ISSN0003-4932.PMC1877027.PMID17435555.
- ^Larkin JO, Collins CG, Aarons S, Tangney M, Whelan M, O'Reily S, Breathnach O, Soden DM, O'Sullivan GC (March 2007)."Electrochemotherapy: aspects of preclinical development and early clinical experience".Annals of Surgery.245(3): 469–79.doi:10.1097/01.sla.0000250419.36053.33.PMC1877027.PMID17435555.
- ^Testori A, Tosti G, Martinoli C, Spadola G, Cataldo F, Verrecchia F, Baldini F, Mosconi M, Soteldo J, Tedeschi I, Passoni C, Pari C, Di Pietro A, Ferrucci PF (2010)."Electrochemotherapy for cutaneous and subcutaneous tumor lesions: a novel therapeutic approach".Dermatologic Therapy.23(6): 651–61.doi:10.1111/j.1529-8019.2010.01370.x.PMID21054709.S2CID46534637.
- ^Marty M, Sersa G, Garbay JR, Gehl J, Collins CG, Snoj M, Billard V, Geertsen PF, Larkin JO, Miklavcic D, Pavlovic I, Paulin-Kosir SM, Cemazar M, Morsli N, Soden DM, Rudolf Z, Robert C, O'Sullivan GC, Mir LM (2006). "Electrochemotherapy – An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases".Eur J Cancer Suppl.4(11): 3–13.doi:10.1016/j.ejcsup.2006.08.002.
- ^Mir LM, Gehl J, Sersa G, Collins CG, Garbay JR, Billard V, Geertsen PF, Rudolf Z, O'Sullivan GC, Marty M (2006). "Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the Cliniporator™ by means of invasive or non-invasive electrodes".Eur J Cancer Suppl.4(11): 14–25.doi:10.1016/j.ejcsup.2006.08.003.
- ^Soden DM, Larkin JO, Collins CG, Tangney M, Aarons S, Piggott J, Morrissey A, Dunne C, O'Sullivan GC (February 2006). "Successful application of targeted electrochemotherapy using novel flexible electrodes and low dose bleomycin to solid tumours".Cancer Letters.232(2): 300–10.doi:10.1016/j.canlet.2005.03.057.PMID15964138.
- ^Miklavcic D, Snoj M, Zupanic A, Kos B, Cemazar M, Kropivnik M, Bracko M, Pecnik T, Gadzijev E, Sersa G (February 2010)."Towards treatment planning and treatment of deep-seated solid tumors by electrochemotherapy".BioMedical Engineering OnLine.9(1): 10.doi:10.1186/1475-925X-9-10.PMC2843684.PMID20178589.
- ^Issels R (1999)."Hyperthermia Combined with Chemotherapy – Biological Rationale, Clinical Application, and Treatment Results".Onkologie.22(5): 374–381.doi:10.1159/000026986.S2CID37581171.
- ^McKnight JA (May 2003). "Principles of chemotherapy".Clinical Techniques in Small Animal Practice.18(2): 67–72.doi:10.1053/svms.2003.36617.PMID12831063.S2CID39060053.
External links
edit- Chemotherapy,American Cancer Society
- Hazardous Drug Exposures in Health Care,National Institute for Occupational Safety and Health
- NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016, National Institute for Occupational Safety and Health
- Wikiversity page for the International Ototoxicity Management Group:https://en.wikiversity.org/wiki/International_Ototoxicity_Management_Group_(IOMG)