Antiviral proteinsareproteinsthat are induced by human or animalcellsto interfere withviral replication.These proteins are isolated to inhibit thevirusfrom replicating in a host's cells and stop it from spreading to other cells.[citation needed]ThePokeweedantiviralproteinand the Zinc-Finger antiviral protein are two major antiviral proteins that have undergone several tests for viruses, includingHIVandinfluenza.[citation needed]
Pokeweed antiviral protein
editPokeweed antiviral protein is aribosome inactivating proteinthat providespokeweedplants protection against both viral and fungal infections.[1]It also protects other types of plants that have genetically engineered to express RAP that do not normally do so.[1]Recombinantpokeweed antiviral protein has also been proposed as a treatment of human diseases such asAIDSand cancer.[2][3]
ZC3HAV1
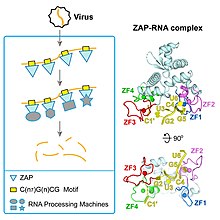
editZAP (Zinc finger Antiviral Protein) is encoded by theZC3HAV1gene in humans[4]whose expression is induced byinterferonand helps fight a number of viral infections including influenza.[5]
RNase L
editRibonuclease LorRNase L(forlatent), ais aninterferon (IFN)-inducedribonucleasewhich, upon activation, destroys allRNAwithin thecell(both cellular and viral) as well as inhibiting mRNA export.[6][7]RNase L is anenzymethat in humans is encoded by theRNASELgenein humans.
IFITM3
editInterferon-induced transmembrane protein 3 (IFITM3) inhibits the replication of number of enveloped RNA viruses includinginfluenza A,HIVand theEbolaandDengueviruses.[8]Consequently pharmacological induction of IFITM3 potentially could be used to treat a number of viral infections.[5]
Protein kinase R
editProtein kinase Ris interferon stimulated and activated either bydouble-stranded RNA(occurring as an intermediate in RNA viruses replication) or by other proteins. It is able to phosphorylate the eukaryotic translation initiation factor eIF2α thus inhibiting further cellular mRNA translation.[9]
References
edit- ^abDi R, Tumer NE (March 2015)."Pokeweed antiviral protein: its cytotoxicity mechanism and applications in plant disease resistance".Toxins.7(3): 755–72.doi:10.3390/toxins7030755.PMC4379523.PMID25756953.
- ^Rajamohan F, Engstrom CR, Denton TJ, Engen LA, Kourinov I, Uckun FM (July 1999). "High-level expression and purification of biologically active recombinant pokeweed antiviral protein".Protein Expression and Purification.16(2): 359–68.doi:10.1006/prep.1999.1084.PMID10419833.
- ^Uckun FM, Rajamohan F, Pendergrass S, Ozer Z, Waurzyniak B, Mao C (March 2003)."Structure-based design and engineering of a nontoxic recombinant pokeweed antiviral protein with potent anti-human immunodeficiency virus activity".Antimicrobial Agents and Chemotherapy.47(3): 1052–61.doi:10.1128/aac.47.3.1052-1061.2003.PMC149289.PMID12604541.
- ^Gupte R, Liu Z, Kraus WL (January 2017)."PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes".Genes & Development.31(2): 101–126.doi:10.1101/gad.291518.116.PMC5322727.PMID28202539.
- ^abBedford JG, O'Keeffe M, Reading PC, Wakim LM (2019)."Rapid interferon independent expression of IFITM3 following T cell activation protects cells from influenza virus infection".PLOS ONE.14(1): e0210132.Bibcode:2019PLoSO..1410132B.doi:10.1371/journal.pone.0210132.PMC6334895.PMID30650117.
- ^Brennan-Laun, Sarah E.; Ezelle, Heather J.; Li, Xiao-Ling; Hassel, Bret A. (April 2014)."RNase-L Control of Cellular mRNAs: Roles in Biologic Functions and Mechanisms of Substrate Targeting".Journal of Interferon & Cytokine Research.34(4): 275–288.doi:10.1089/jir.2013.0147.ISSN1079-9907.PMC3976596.PMID24697205.
- ^Burke, James M.; Gilchrist, Alison R.; Sawyer, Sara L.; Parker, Roy (2021-06-04)."RNase L limits host and viral protein synthesis via inhibition of mRNA export".Science Advances.7(23).doi:10.1126/sciadv.abh2479.ISSN2375-2548.PMC8177694.PMID34088676.
- ^Wellington D, Laurenson-Schafer H, Abdel-Haq A, Dong T (February 2019)."IFITM3: How genetics influence influenza infection demographically".Biomedical Journal.42(1): 19–26.doi:10.1016/j.bj.2019.01.004.PMC6468115.PMID30987701.
- ^Fensterl, V.; Sen, G. C. (2009),"Interferons and viral infections",BioFactors,35(1): 14–20,doi:10.1002/biof.6,PMID19319841,S2CID27209861