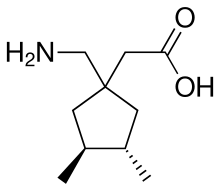
Atagabalin(PD-0200,390) is a drug developed byPfizerand related togabapentin,which similarly binds to the α2δcalcium channels(1and2).[1]It was under development as a treatment forinsomnia,[2][3][4]but was discontinued following unsatisfactory trial results.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
| Formula | C10H19NO2 |
| Molar mass | 185.267g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
editReferences
edit- ^Blakemore DC, Bryans JS, Carnell P, Carr CL, Chessum NE, Field MJ, Kinsella N, Osborne SA, Warren AN, Williams SC (January 2010). "Synthesis and in vivo evaluation of bicyclic gababutins".Bioorganic & Medicinal Chemistry Letters.20(2): 461–4.doi:10.1016/j.bmcl.2009.11.118.PMID20005103.
- ^Corrigan B, Feltner DE, Ouellet D, Werth JL, Moton AE, Gibson G (August 2009)."Effect of renal impairment on the pharmacokinetics of PD 0200390, a novel ligand for the voltage-gated calcium channel alpha-2-delta subunit".British Journal of Clinical Pharmacology.68(2): 174–80.doi:10.1111/j.1365-2125.2009.03444.x.PMC2767279.PMID19694735.
- ^Quintero JE, Pomerleau F, Huettl P, Johnson KW, Offord J, Gerhardt GA (May 2011)."Methodology for rapid measures of glutamate release in rat brain slices using ceramic-based microelectrode arrays: Basic characterization and drug pharmacology".Brain Research.1401:1–9.doi:10.1016/j.brainres.2011.05.025.PMC3197737.PMID21664606.
- ^Kjellsson MC, Ouellet D, Corrigan B, Karlsson MO (June 2011). "Modeling Sleep Data for a New Drug in Development using Markov Mixed-Effects Models".Pharmaceutical Research.28(10): 2610–27.doi:10.1007/s11095-011-0490-x.PMID21681607.S2CID22241527.