Abacteriophage(/bækˈtɪərioʊfeɪdʒ/), also known informally as aphage(/ˈfeɪdʒ/), is avirusthat infects and replicates withinbacteriaandarchaea.The term is derived fromAncient Greekφαγεῖν(phagein)'to devour' andbacteria.Bacteriophages are composed ofproteinsthatencapsulateaDNAorRNAgenome,and may have structures that are either simple or elaborate. Their genomes may encode as few as four genes (e.g.MS2) and as many as hundreds ofgenes.Phages replicate within the bacterium following the injection of their genome into itscytoplasm.

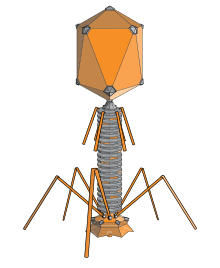

Bacteriophages are among the most common and diverse entities in thebiosphere.[2]Bacteriophages are ubiquitous viruses, found wherever bacteria exist. It is estimated there are more than 1031bacteriophages on the planet, more than every other organism on Earth, including bacteria, combined.[3]Viruses are the most abundant biological entity in the water column of the world's oceans, and the second largest component of biomass afterprokaryotes,[4]where up to 9x108virionsper millilitre have been found inmicrobial matsat the surface,[5]and up to 70% ofmarine bacteriamay be infected by bacteriophages.[6]
Bacteriophages were used from the 1920s as an alternative toantibioticsin the formerSoviet Unionand Central Europe, as well as in France.[7][8]They are seen as a possible therapy againstmulti-drug-resistantstrains of many bacteria (seephage therapy).[9][10][11][12]
Bacteriophages are known to interact with the immune system both indirectly via bacterial expression of phage-encoded proteins and directly by influencing innate immunity and bacterial clearance.[13]Phage–host interactions are becoming increasingly important areas of research.[14]
Classification
editBacteriophages occur abundantly in the biosphere, with different genomes and lifestyles. Phages are classified by theInternational Committee on Taxonomy of Viruses(ICTV) according tomorphologyand nucleic acid.
| Order | Family | Morphology | Nucleic acid | Examples |
|---|---|---|---|---|
| Belfryvirales | Turriviridae | Enveloped, isometric | Linear dsDNA | |
| Caudovirales | Ackermannviridae | Nonenveloped,contractile tail | Linear dsDNA | |
| Autographiviridae | Nonenveloped, noncontractile tail (short) | Linear dsDNA | ||
| Chaseviridae | Linear dsDNA | |||
| Demerecviridae | Linear dsDNA | |||
| Drexlerviridae | Linear dsDNA | |||
| Guenliviridae | Linear dsDNA | |||
| Herelleviridae | Nonenveloped, contractile tail | Linear dsDNA | ||
| Myoviridae | Nonenveloped, contractile tail | Linear dsDNA | T4,Mu,P1,P2 | |
| Siphoviridae | Nonenveloped, noncontractile tail (long) | Linear dsDNA | λ,T5,HK97,N15 | |
| Podoviridae | Nonenveloped, noncontractile tail (short) | Linear dsDNA | T7,T3,Φ29,P22 | |
| Rountreeviridae | Linear dsDNA | |||
| Salasmaviridae | Linear dsDNA | |||
| Schitoviridae | Linear dsDNA | |||
| Zobellviridae | Linear dsDNA | |||
| Halopanivirales | Sphaerolipoviridae | Enveloped, isometric | Linear dsDNA | |
| Simuloviridae | Enveloped, isometric | Linear dsDNA | ||
| Matshushitaviridae | Enveloped, isometric | Linear dsDNA | ||
| Haloruvirales | Pleolipoviridae | Enveloped, pleomorphic | Circular ssDNA, circular dsDNA, or linear dsDNA | |
| Kalamavirales | Tectiviridae | Nonenveloped, isometric | Linear dsDNA | |
| Ligamenvirales | Lipothrixviridae | Enveloped, rod-shaped | Linear dsDNA | Acidianus filamentous virus 1 |
| Rudiviridae | Nonenveloped, rod-shaped | Linear dsDNA | Sulfolobus islandicus rod-shaped virus 1 | |
| Mindivirales | Cystoviridae | Enveloped, spherical | Linear dsRNA | Φ6 |
| Norzivirales | Atkinsviridae | Nonenveloped, isometric | Linear ssRNA | |
| Duinviridae | Nonenveloped, isometric | Linear ssRNA | ||
| Fiersviridae | Nonenveloped, isometric | Linear ssRNA | MS2,Qβ | |
| Solspiviridae | Nonenveloped, isometric | Linear ssRNA | ||
| Petitvirales | Microviridae | Nonenveloped, isometric | Circular ssDNA | ΦX174 |
| Primavirales | Tristromaviridae | Enveloped, rod-shaped | Linear dsDNA | |
| Timlovirales | Blumeviridae | Nonenveloped, isometric | Linear ssRNA | |
| Steitzviridae | Nonenveloped, isometric | Linear ssRNA | ||
| Tubulavirales | Inoviridae | Nonenveloped, filamentous | Circular ssDNA | M13 |
| Paulinoviridae | Nonenveloped, filamentous | Circular ssDNA | ||
| Plectroviridae | Nonenveloped, filamentous | Circular ssDNA | ||
| Vinavirales | Corticoviridae | Nonenveloped, isometric | Circular dsDNA | PM2 |
| Durnavirales | Picobirnaviridae(proposal) | Nonenveloped, isometric | Linear dsRNA | |
| Unassigned | Ampullaviridae | Enveloped, bottle-shaped | Linear dsDNA | |
| Autolykiviridae | Nonenveloped, isometric | Linear dsDNA | ||
| Bicaudaviridae | Nonenveloped, lemon-shaped | Circular dsDNA | ||
| Clavaviridae | Nonenveloped, rod-shaped | Circular dsDNA | ||
| Finnlakeviridae | Nonenveloped, isometric | Circular ssDNA | FLiP[15] | |
| Fuselloviridae | Nonenveloped, lemon-shaped | Circular dsDNA | Alphafusellovirus | |
| Globuloviridae | Enveloped, isometric | Linear dsDNA | ||
| Guttaviridae | Nonenveloped, ovoid | Circular dsDNA | ||
| Halspiviridae | Nonenveloped, lemon-shaped | Linear dsDNA | ||
| Plasmaviridae | Enveloped, pleomorphic | Circular dsDNA | ||
| Portogloboviridae | Enveloped, isometric | Circular dsDNA | ||
| Thaspiviridae | Nonenveloped, lemon-shaped | Linear dsDNA | ||
| Spiraviridae | Nonenveloped, rod-shaped | Circular ssDNA |
It has been suggested that members ofPicobirnaviridaeinfect bacteria, but not mammals.[16]
There are also many unassigned genera of the classLeviviricetes:Chimpavirus,Hohglivirus,Mahrahvirus,Meihzavirus,Nicedsevirus,Sculuvirus,Skrubnovirus,TetipavirusandWinunaviruscontaining linear ssRNA genomes[17]and the unassigned genusLilyvirusof the orderCaudoviralescontaining a linear dsDNA genome.
History
editIn 1896,Ernest Hanbury Hankinreported that something in the waters of theGangesandYamunarivers inIndiahad a marked antibacterial action againstcholeraand it could pass through a very fine porcelain filter.[18]In 1915,BritishbacteriologistFrederick Twort,superintendent of the Brown Institution of London, discovered a small agent that infected and killed bacteria. He believed the agent must be one of the following:
- a stage in thelife cycleof the bacteria
- anenzymeproduced by the bacteria themselves, or
- a virus that grew on and destroyed the bacteria[19]
Twort's research was interrupted by the onset ofWorld War I,as well as a shortage of funding and the discoveries of antibiotics.
Independently,French-CanadianmicrobiologistFélix d'Hérelle,working at thePasteur InstituteinParis,announced on 3 September 1917 that he had discovered "an invisible, antagonistic microbe of thedysenterybacillus ". For d'Hérelle, there was no question as to the nature of his discovery:" In a flash I had understood: what caused my clear spots was in fact an invisible microbe... a virus parasitic on bacteria. "[20]D'Hérelle called the virus a bacteriophage, a bacterium-eater (from the Greekphagein,meaning "to devour" ). He also recorded a dramatic account of a man suffering from dysentery who was restored to good health by the bacteriophages.[21]It was d'Hérelle who conducted much research into bacteriophages and introduced the concept ofphage therapy.[22]In 1919, in Paris, France, d'Hérelle conducted the first clinical application of a bacteriophage, with the first reported use in theUnited Statesbeing in 1922.[23]
Nobel prizes awarded for phage research
editIn 1969,Max Delbrück,Alfred Hershey,andSalvador Luriawere awarded theNobel Prize in Physiology or Medicinefor their discoveries of the replication of viruses and their genetic structure.[24]Specifically the work of Hershey, as contributor to theHershey–Chase experimentin 1952, provided convincing evidence that DNA, not protein, was the genetic material of life. Delbrück and Luria carried out theLuria–Delbrück experimentwhich demonstrated statistically that mutations in bacteria occur randomly and thus followDarwinianrather thanLamarckianprinciples.
Uses
editPhage therapy
editPhages were discovered to be antibacterial agents and were used in the formerSovietRepublic ofGeorgia(pioneered there byGiorgi Eliavawith help from the co-discoverer of bacteriophages,Félix d'Hérelle) during the 1920s and 1930s for treating bacterial infections.
D'Herelle "quickly learned that bacteriophages are found wherever bacteria thrive: in sewers, in rivers that catch waste runoff from pipes, and in the stools of convalescent patients."[25]
They had widespread use, including treatment of soldiers in theRed Army.[26]However, they were abandoned for general use in the West for several reasons:
- Antibiotics were discovered and marketed widely. They were easier to make, store, and prescribe.
- Medical trials of phages were carried out, but a basic lack of understanding of phages raised questions about the validity of these trials.[27]
- Publication of research in the Soviet Union was mainly in theRussianorGeorgian languagesand for many years was not followed internationally.
- The Soviet technology was widely discouraged and in some cases illegal due to thered scare.
The use of phages has continued since the end of theCold Warin Russia,[28]Georgia, and elsewhere in Central and Eastern Europe. The first regulated, randomized, double-blindclinical trialwas reported in theJournal of Wound Carein June 2009, which evaluated the safety and efficacy of a bacteriophage cocktail to treat infected venous ulcers of the leg in human patients.[29]The FDA approved the study as a Phase I clinical trial. The study's results demonstrated the safety of therapeutic application of bacteriophages, but did not show efficacy. The authors explained that the use of certain chemicals that are part of standard wound care (e.g.lactoferrinor silver) may have interfered with bacteriophage viability.[29]Shortly after that, another controlled clinical trial in Western Europe (treatment of ear infections caused byPseudomonas aeruginosa) was reported in the journalClinical Otolaryngologyin August 2009.[30]The study concludes that bacteriophage preparations were safe and effective for treatment of chronic ear infections in humans. Additionally, there have been numerous animal and other experimental clinical trials evaluating the efficacy of bacteriophages for various diseases, such as infected burns and wounds, and cystic fibrosis-associated lung infections, among others.[30]On the other hand, phages ofInoviridaehave been shown to complicatebiofilmsinvolved inpneumoniaandcystic fibrosisand to shelter the bacteria from drugs meant to eradicate disease, thus promoting persistent infection.[31]
Meanwhile, bacteriophage researchers have been developing engineered viruses to overcomeantibiotic resistance,and engineering the phage genes responsible for coding enzymes that degrade the biofilm matrix, phage structural proteins, and the enzymes responsible forlysisof the bacterial cell wall.[5][6][7]There have been results showing that T4 phages that are small in size and short-tailed can be helpful in detectingE. coliin the human body.[32]
Therapeutic efficacy of a phage cocktail was evaluated in a mouse model with nasal infection of multi-drug-resistant (MDR)A. baumannii.Mice treated with the phage cocktail showed a 2.3-fold higher survival rate compared to those untreated at seven days post-infection.[33]
In 2017, a 68-year-old diabetic patient with necrotizing pancreatitis complicated by a pseudocyst infected with MDRA. baumanniistrains was being treated with a cocktail of Azithromycin, Rifampicin, and Colistin for 4 months without results and overall rapidly declining health.
Because discussion had begun of the clinical futility of further treatment, an Emergency Investigational New Drug (eIND) was filed as a last effort to at the very least gain valuable medical data from the situation, and approved, so he was subjected to phage therapy using a percutaneously (PC) injected cocktail containing nine different phages that had been identified as effective against the primary infection strain by rapid isolation and testing techniques (a process which took under a day). This proved effective for a very brief period, although the patient remained unresponsive and his health continued to worsen; soon isolates of a strain ofA. baumanniiwere being collected from drainage of the cyst that showed resistance to this cocktail, and a second cocktail which was tested to be effective against this new strain was added, this time by intravenous (IV) injection as it had become clear that the infection was more pervasive than originally thought.[34]
Once on the combination of the IV and PC therapy the patient's downward clinical trajectory reversed, and within two days he had awoken from his coma and become responsive. As his immune system began to function he had to be temporarily removed from the cocktail because his fever was spiking to over 104 °F (40 °C), but after two days the phage cocktails were re-introduced at levels he was able to tolerate. The original three-antibiotic cocktail was replaced by minocycline after the bacterial strain was found not to be resistant to this and he rapidly regained full lucidity, although he was not discharged from the hospital until roughly 145 days after phage therapy began. Towards the end of the therapy it was discovered that the bacteria had become resistant to both of the original phage cocktails, but they were continued because they seemed to be preventing minocycline resistance from developing in the bacterial samples collected so were having a useful synergistic effect.[34]
Other
editFood industry
editPhages have increasingly been used to safen food products and to forestallspoilage bacteria.[35]Since 2006, theUnited States Food and Drug Administration(FDA) andUnited States Department of Agriculture(USDA) have approved several bacteriophage products. LMP-102 (Intralytix) was approved for treating ready-to-eat (RTE) poultry and meat products. In that same year, the FDA approved LISTEX (developed and produced byMicreos) using bacteriophages on cheese to killListeria monocytogenesbacteria, in order to give themgenerally recognized as safe(GRAS) status.[36]In July 2007, the same bacteriophage were approved for use on all food products.[37]In 2011 USDA confirmed that LISTEX is a clean label processing aid and is included in USDA.[38]Research in the field of food safety is continuing to see if lytic phages are a viable option to control other food-borne pathogens in various food products.[39]
Water indicators
editBacteriophages, including those specific toEscherichia coli,have been employed as indicators of fecal contamination in water sources. Due to their shared structural and biological characteristics, coliphages can serve as proxies for viral fecal contamination and the presence of pathogenic viruses such as rotavirus, norovirus, and HAV. Research conducted on wastewater treatment systems has revealed significant disparities in the behavior of coliphages compared to fecal coliforms, demonstrating a distinct correlation with the recovery of pathogenic viruses at the treatment's conclusion. Establishing a secure discharge threshold, studies have determined that discharges below 3000 PFU/100 mL are considered safe in terms of limiting the release of pathogenic viruses.[40]
Diagnostics
editIn 2011, the FDA cleared the first bacteriophage-based product for in vitro diagnostic use.[41]The KeyPath MRSA/MSSA Blood Culture Test uses a cocktail of bacteriophage to detectStaphylococcus aureusin positive blood cultures and determinemethicillinresistance or susceptibility. The test returns results in about five hours, compared to two to three days for standard microbial identification and susceptibility test methods. It was the first accelerated antibiotic-susceptibility test approved by the FDA.[42]
Counteracting bioweapons and toxins
editGovernment agencies in the West have for several years been looking toGeorgiaand the formerSoviet Unionfor help with exploiting phages for counteracting bioweapons and toxins, such asanthraxandbotulism.[43]Developments are continuing among research groups in the U.S. Other uses include spray application in horticulture for protecting plants and vegetable produce from decay and the spread of bacterial disease. Other applications for bacteriophages are as biocides for environmental surfaces, e.g., in hospitals, and as preventative treatments for catheters and medical devices before use in clinical settings. The technology for phages to be applied to dry surfaces, e.g., uniforms, curtains, or even sutures for surgery now exists. Clinical trials reported inClinical Otolaryngology[30]show success in veterinary treatment of pet dogs withotitis.
Bacterium sensing and identification
editThesensing of phage-triggered ion cascades(SEPTIC) bacterium sensing and identification method uses the ion emission and its dynamics during phage infection and offers high specificity and speed for detection.[44]
Phage display
editPhage displayis a different use of phages involving a library of phages with a variable peptide linked to a surface protein. Each phage genome encodes the variant of the protein displayed on its surface (hence the name), providing a link between the peptide variant and its encoding gene. Variant phages from the library may be selected through their binding affinity to an immobilized molecule (e.g., botulism toxin) to neutralize it. The bound, selected phages can be multiplied by reinfecting a susceptible bacterial strain, thus allowing them to retrieve the peptides encoded in them for further study.[45]
Antimicrobial drug discovery
editPhage proteins often have antimicrobial activity and may serve as leads forpeptidomimetics,i.e. drugs that mimic peptides.[46]Phage-ligand technologymakes use of phage proteins for various applications, such as binding of bacteria and bacterial components (e.g.endotoxin) and lysis of bacteria.[47]
Basic research
editBacteriophages are importantmodel organismsfor studying principles ofevolutionandecology.[48]
Detriments
editDairy industry
editBacteriophages present in the environment can cause cheese to not ferment. In order to avoid this, mixed-strain starter cultures and culture rotation regimes can be used.[49]Genetic engineeringof culture microbes – especiallyLactococcus lactisandStreptococcus thermophilus– have been studied for genetic analysis and modification to improvephage resistance.This has especially focused onplasmidandrecombinantchromosomal modifications.[50][35]
Some research has focused on the potential of bacteriophages as antimicrobial against foodborne pathogens and biofilm formation within the dairy industry. As the spread of antibiotic resistance is a main concern within the dairy industry, phages can serve as a promising alternative.[51]
Replication
editThe life cycle of bacteriophages tends to be either alytic cycleor alysogenic cycle.In addition, some phages display pseudolysogenic behaviors.[13]
Withlytic phagessuch as theT4 phage,bacterial cells are broken open (lysed) and destroyed after immediate replication of the virion. As soon as the cell is destroyed, the phage progeny can find new hosts to infect.[13]Lytic phages are more suitable forphage therapy.Some lytic phages undergo a phenomenon known as lysis inhibition, where completed phage progeny will not immediately lyse out of the cell if extracellular phage concentrations are high. This mechanism is not identical to that of thetemperate phagegoing dormant and usually is temporary.[52]
In contrast, thelysogenic cycledoes not result in immediate lysing of the host cell. Those phages able to undergo lysogeny are known astemperate phages.Their viral genome will integrate with host DNA and replicate along with it, relatively harmlessly, or may even become established as aplasmid.The virus remains dormant until host conditions deteriorate, perhaps due to depletion of nutrients, then, theendogenousphages (known asprophages) become active. At this point they initiate the reproductive cycle, resulting in lysis of the host cell. As the lysogenic cycle allows the host cell to continue to survive and reproduce, the virus is replicated in all offspring of the cell. An example of a bacteriophage known to follow the lysogenic cycle and the lytic cycle is thephage lambdaofE. coli.[53]
Sometimes prophages may provide benefits to the host bacterium while they are dormant by adding new functions to the bacterialgenome,in a phenomenon calledlysogenic conversion.Examples are the conversion of harmless strains ofCorynebacterium diphtheriaeorVibrio choleraeby bacteriophages to highly virulent ones that causediphtheriaorcholera,respectively.[54][55]Strategies to combat certain bacterial infections by targeting these toxin-encoding prophages have been proposed.[56]
Attachment and penetration
editBacterial cells are protected by a cell wall ofpolysaccharides,which are important virulence factors protecting bacterial cells against both immune host defenses andantibiotics.[57]To enter a host cell, bacteriophages bind to specific receptors on the surface of bacteria, includinglipopolysaccharides,teichoic acids,proteins,or evenflagella.This specificity means a bacteriophage can infect only certain bacteria bearing receptors to which they can bind, which in turn, determines the phage's host range. Polysaccharide-degrading enzymes are virion-associated proteins that enzymatically degrade the capsular outer layer of their hosts at the initial step of a tightly programmed phage infection process.[citation needed] Host growth conditions also influence the ability of the phage to attach and invade them.[58]As phage virions do not move independently, they must rely on random encounters with the correct receptors when in solution, such as blood, lymphatic circulation, irrigation, soil water, etc.[citation needed]
Myovirus bacteriophages use ahypodermic syringe-like motion to inject their genetic material into the cell. After contacting the appropriate receptor, the tail fibers flex to bring the base plate closer to the surface of the cell. This is known as reversible binding. Once attached completely, irreversible binding is initiated and the tail contracts, possibly with the help ofATPpresent in the tail,[6]injecting genetic material through the bacterial membrane.[59]The injection is accomplished through a sort of bending motion in the shaft by going to the side, contracting closer to the cell and pushing back up. Podoviruses lack an elongated tail sheath like that of a myovirus, so instead, they use their small, tooth-like tail fibers enzymatically to degrade a portion of the cell membrane before inserting their genetic material.
Synthesis of proteins and nucleic acid
editWithin minutes, bacterialribosomesstart translating viral mRNA into protein. For RNA-based phages,RNA replicaseis synthesized early in the process. Proteins modify the bacterialRNA polymeraseso it preferentially transcribes viral mRNA. The host's normal synthesis of proteins and nucleic acids is disrupted, and it is forced to manufacture viral products instead. These products go on to become part of new virions within the cell, helper proteins that contribute to the assemblage of new virions, or proteins involved in celllysis.In 1972,Walter Fiers(University of Ghent,Belgium) was the first to establish the complete nucleotide sequence of a gene and in 1976, of the viral genome ofbacteriophage MS2.[60]SomedsDNAbacteriophages encode ribosomal proteins, which are thought to modulate protein translation during phage infection.[61]
Virion assembly
editIn the case of theT4 phage,the construction of new virus particles involves the assistance of helper proteins that act catalytically during phagemorphogenesis.[62]The base plates are assembled first, with the tails being built upon them afterward. The head capsids, constructed separately, will spontaneously assemble with the tails. During assembly of thephage T4virion,the morphogenetic proteins encoded by the phagegenesinteract with each other in a characteristic sequence. Maintaining an appropriate balance in the amounts of each of these proteins produced during viral infection appears to be critical for normal phage T4morphogenesis.[63]The DNA is packed efficiently within the heads.[64]The whole process takes about 15 minutes.
Early studies of bactioriophage T4 (1962-1964) provided an opportunity to gain understanding of virtually all of the genes that are essential for growth of the bacteriophage under laboratory conditions.[65][66]These studies were made possible by the availability of two classes ofconditional lethal mutants.[67]One class of such mutants was referred to asamber mutants.[67]The other class of conditional lethal mutants was referred to astemperature-sensitive mutants[68]Studies of these two classes of mutants led to considerable insight into the functions and interactions of the proteins employed in the machinery ofDNA replication,repairandrecombination,and on how viruses are assembled from protein and nucleic acid components (molecularmorphogenesis).
Release of virions
editPhages may be released via cell lysis, by extrusion, or, in a few cases, by budding. Lysis, by tailed phages, is achieved by an enzyme calledendolysin,which attacks and breaks down the cell wallpeptidoglycan.An altogether different phage type, thefilamentous phage,makes the host cell continually secrete new virus particles. Released virions are described as free, and, unless defective, are capable of infecting a new bacterium. Budding is associated with certainMycoplasmaphages. In contrast to virion release, phages displaying alysogeniccycle do not kill the host and instead become long-term residents asprophages.[69]
Communication
editResearch in 2017 revealed that the bacteriophage Φ3T makes a short viral protein that signals other bacteriophages to lie dormant instead of killing the host bacterium.Arbitriumis the name given to this protein by the researchers who discovered it.[70][71]
Genome structure
editGiven the millions of different phages in the environment, phage genomes come in a variety of forms and sizes. RNA phages such asMS2have the smallest genomes, with only a few kilobases. However, some DNA phages such asT4may have large genomes with hundreds of genes; the size and shape of thecapsidvaries along with the size of the genome.[72]The largest bacteriophage genomes reach a size of 735 kb.[73]
Bacteriophage genomes can be highlymosaic,i.e. the genome of many phage species appear to be composed of numerous individual modules. These modules may be found in other phage species in different arrangements.Mycobacteriophages,bacteriophages withmycobacterialhosts, have provided excellent examples of this mosaicism. In these mycobacteriophages, genetic assortment may be the result of repeated instances ofsite-specific recombinationandillegitimate recombination(the result of phage genome acquisition of bacterial host genetic sequences).[75]Evolutionary mechanisms shaping the genomes of bacterial viruses vary between different families and depend upon the type of the nucleic acid, characteristics of the virion structure, as well as the mode of the viral life cycle.[76]
Some marineroseobacterphages containdeoxyuridine(dU) instead ofdeoxythymidine(dT) in their genomic DNA. There is some evidence that this unusual component is a mechanism to evade bacterial defense mechanisms such asrestriction endonucleasesandCRISPR/Cassystems which evolved to recognize and cleave sequences within invading phages, thereby inactivating them. Other phages have long been known to use unusual nucleotides. In 1963, Takahashi and Marmur identified aBacillusphage that has dU substituting dT in its genome,[77]and in 1977, Kirnos et al. identified acyanophagecontaining 2-aminoadenine (Z) instead of adenine (A).[78]
Systems biology
editThe field ofsystems biologyinvestigates the complexnetworks of interactionswithin an organism, usually using computational tools and modeling.[79]For example, a phage genome that enters into a bacterial host cell may express hundreds of phage proteins which will affect the expression of numerous host genes or the host'smetabolism.All of these complex interactions can be described and simulated in computer models.[79]
For instance, infection ofPseudomonas aeruginosaby the temperate phage PaP3 changed the expression of 38% (2160/5633) of its host's genes. Many of these effects are probably indirect, hence the challenge becomes to identify the direct interactions among bacteria and phage.[80]
Several attempts have been made to mapprotein–protein interactionsamong phage and their host. For instance, bacteriophage lambda was found to interact with its host,E. coli,by dozens of interactions. Again, the significance of many of these interactions remains unclear, but these studies suggest that there most likely are several key interactions and many indirect interactions whose role remains uncharacterized.[81]
Host resistance
editBacteriophages are a major threat to bacteria and prokaryotes have evolved numerous mechanisms to block infection or to block the replication of bacteriophages within host cells. TheCRISPR systemis one such mechanism as areretronsand the anti-toxin system encoded by them.[82]The Thoeris defense system is known to deploy a unique strategy for bacterial antiphage resistance viaNAD+degradation.[83]
Bacteriophage–host symbiosis
editTemperate phages are bacteriophages that integrate their genetic material into the host as extrachromosomal episomes or as aprophageduring alysogenic cycle.[84][85][86]Some temperate phages can confer fitness advantages to their host in numerous ways, including giving antibiotic resistance through the transfer or introduction of antibiotic resistance genes (ARGs),[85][87]protecting hosts from phagocytosis,[88][89]protecting hosts from secondary infection through superinfection exclusion,[90][91][92]enhancing host pathogenicity,[84][93]or enhancing bacterial metabolism or growth.[94][95][96][97]Bacteriophage–host symbiosis may benefit bacteria by providing selective advantages while passively replicating the phage genome.[98]
In the environment
editMetagenomicshas allowed the in-water detection of bacteriophages that was not possible previously.[99]
Also, bacteriophages have been used inhydrologicaltracing and modelling inriversystems, especially where surface water andgroundwaterinteractions occur. The use of phages is preferred to the more conventionaldyemarker because they are significantly less absorbed when passing through ground waters and they are readily detected at very low concentrations.[100]Non-polluted water may contain approximately 2×108bacteriophages per ml.[101]
Bacteriophages are thought to contribute extensively tohorizontal gene transferin natural environments, principally viatransduction,but also viatransformation.[102]Metagenomics-based studies also have revealed thatviromesfrom a variety of environments harbor antibiotic-resistance genes, including those that could confermultidrug resistance.[103]
Recent findings have mapped the complex and intertwined arsenal of anti-phage defense tools in environmental bacteria.[104]
In humans
editAlthough phages do not infect humans, there are countless phage particles in the human body, given the extensivehuman microbiome.One's phage population has been called the humanphageome,including the "healthy gut phageome" (HGP) and the "diseased human phageome" (DHP).[105]The active phageome of a healthy human (i.e., actively replicating as opposed to nonreplicating, integratedprophage) has been estimated to comprise dozens to thousands of different viruses.[106] There is evidence that bacteriophages and bacteria interact in thehuman gut microbiomeboth antagonistically and beneficially.[107]
Preliminary studies have indicated that common bacteriophages are found in 62% of healthy individuals on average, while their prevalence was reduced by 42% and 54% on average in patients withulcerative colitis(UC) andCrohn's disease(CD).[105]Abundance of phages may also decline in the elderly.[107]
The most common phages in the human intestine, found worldwide, arecrAssphages.CrAssphages are transmitted from mother to child soon after birth, and there is some evidence suggesting that they may be transmitted locally. Each person develops their own unique crAssphage clusters. CrAss-like phages also may be present inprimatesbesides humans.[107]
Commonly studied bacteriophages
editAmong the countless phages, only a few have been studied in detail, including some historically important phage that were discovered in the early days of microbial genetics. These, especially the T-phage, helped to discover important principles of gene structure and function.
Bacteriophage databases and resources
editSee also
edit- Antibiotic
- Bacterivore
- CrAssphage
- CRISPR
- DNA viruses
- Macrophage
- Phage ecology
- Phage monographs(a comprehensive listing of phage and phage-associated monographs, 1921–present)
- Phagemid
- Polyphage
- RNA viruses
- Transduction
- Viriome
- Virophage,viruses that infect other viruses
References
edit- ^Padilla-Sanchez V (2021)."Structural Model of Bacteriophage T4".WikiJournal of Science.4(1): 5.doi:10.15347/WJS/2021.005.S2CID238939621.
- ^abMcGrath S, van Sinderen D, eds. (2007).Bacteriophage: Genetics and Molecular Biology(1st ed.). Caister Academic Press.ISBN978-1-904455-14-1.
- ^LaFee S, Buschman H (25 April 2017)."Novel Phage Therapy Saves Patient with Multidrug-Resistant Bacterial Infection".UC Health – UC San Diego.Retrieved13 May2018.
- ^Suttle CA (September 2005). "Viruses in the sea".Nature.437(7057): 356–361.Bibcode:2005Natur.437..356S.doi:10.1038/nature04160.PMID16163346.S2CID4370363.
- ^abWommack KE, Colwell RR (March 2000)."Virioplankton: viruses in aquatic ecosystems".Microbiology and Molecular Biology Reviews.64(1): 69–114.doi:10.1128/MMBR.64.1.69-114.2000.PMC98987.PMID10704475.
- ^abcm Prescott L (1993).Microbiology.Wm. C. Brown Publishers.ISBN0-697-01372-3.
- ^abBunting J (1997)."The Virus that Cures".BBC Horizon.BBC Worldwide Ltd.OCLC224991186.– Documentary about the history of phage medicine in Russia and the West
- ^Borrell B, Fishchetti V (August 2012). "Science talk: Phage factor".Scientific American.pp. 80–83.JSTOR26016042.
- ^Kortright KE, Chan BK, Koff JL, Turner PE (February 2019)."Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria".Cell Host & Microbe.25(2): 219–232.doi:10.1016/j.chom.2019.01.014.PMID30763536.S2CID73439131.
- ^Gordillo Altamirano FL, Barr JJ (April 2019)."Phage Therapy in the Postantibiotic Era".Clinical Microbiology Reviews.32(2).doi:10.1128/CMR.00066-18.PMC6431132.PMID30651225.
- ^González-Mora A, Hernández-Pérez J, Iqbal HM, Rito-Palomares M, Benavides J (September 2020)."Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery".Vaccines.8(3): 504.doi:10.3390/vaccines8030504.PMC7565293.PMID32899720.
- ^Keen EC (2012)."Phage therapy: concept to cure".Frontiers in Microbiology.3:238.doi:10.3389/fmicb.2012.00238.PMC3400130.PMID22833738.
- ^abcPopescu M, Van Belleghem JD, Khosravi A, Bollyky PL (September 2021)."Bacteriophages and the Immune System".Annual Review of Virology.8(1): 415–435.doi:10.1146/annurev-virology-091919-074551.PMID34014761.
- ^Stone E, Campbell K, Grant I, McAuliffe O (June 2019)."Understanding and Exploiting Phage-Host Interactions".Viruses.11(6): 567.doi:10.3390/v11060567.PMC6630733.PMID31216787.
- ^Laanto E, Mäntynen S, De Colibus L, Marjakangas J, Gillum A, Stuart DI, et al. (August 2017)."Virus found in a boreal lake links ssDNA and dsDNA viruses".Proceedings of the National Academy of Sciences of the United States of America.114(31): 8378–8383.Bibcode:2017PNAS..114.8378L.doi:10.1073/pnas.1703834114.PMC5547622.PMID28716906.
- ^Krishnamurthy SR, Wang D (March 2018)."Extensive conservation of prokaryotic ribosomal binding sites in known and novel picobirnaviruses".Virology.516:108–114.doi:10.1016/j.virol.2018.01.006.PMID29346073.
- ^Callanan J, Stockdale SR, Adriaenssens EM, Kuhn JH (January 2021)."Rename one class (Leviviricetes - formerly Allassoviricetes), rename one order (Norzivirales - formerly Levivirales), create one new order (Timlovirales), and expand the class to a total of six families, 420 genera and 883 species".ResearchGate.doi:10.13140/RG.2.2.25363.40481.
- ^Hankin EH (1896)."L'action bactericide des eaux de la Jumna et du Gange sur le vibrion du cholera".Annales de l'Institut Pasteur(in French).10:511–23.
- ^Twort FW (1915)."An Investigation on the Nature of Ultra-Microscopic Viruses".The Lancet.186(4814): 1241–43.doi:10.1016/S0140-6736(01)20383-3.
- ^d'Hérelles F (1917)."Sur un microbe invisible antagoniste des bacilles dysentériques"(PDF).Comptes Rendus de l'Académie des Sciences de Paris.165:373–5.Archived(PDF)from the original on 11 May 2011.Retrieved5 September2010.
- ^d'Hérelles F (1949)."The bacteriophage"(PDF).Science News.14:44–59.Retrieved5 September2010.
- ^Keen EC (December 2012). "Felix d'Herelle and our microbial future".Future Microbiology.7(12): 1337–1339.doi:10.2217/fmb.12.115.PMID23231482.
- ^Aswani VH, Shukla SK (June 2021)."An Early History of Phage Therapy in the United States: Is it Time to Reconsider?".Clinical Medicine & Research.19(2): 82–89.doi:10.3121/cmr.2021.1605.PMC8231696.PMID34172535.
- ^"The Nobel Prize in Physiology or Medicine 1969".Nobel Foundation.Retrieved28 July2007.
- ^Kuchment A (2012),The Forgotten Cure: The past and future of phage therapy,Springer, p. 11,ISBN978-1-4614-0250-3
- ^Myelnikov D (October 2018)."An Alternative Cure: The Adoption and Survival of Bacteriophage Therapy in the USSR, 1922-1955".Journal of the History of Medicine and Allied Sciences.73(4): 385–411.doi:10.1093/jhmas/jry024.PMC6203130.PMID30312428.
- ^Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, et al. (January 2010). "Phage therapy in clinical practice: treatment of human infections".Current Pharmaceutical Biotechnology.11(1): 69–86.doi:10.2174/138920110790725401.PMID20214609.S2CID31626252.
- ^Golovin S (2017)."Бактериофаги: убийцы в роли спасителей"[Bacteriophages: killers as saviors].Наука и жизнь[Nauka I Zhizn (Science and life)] (in Russian) (6): 26–33.
- ^abRhoads DD, Wolcott RD, Kuskowski MA, Wolcott BM, Ward LS, Sulakvelidze A (June 2009). "Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial".Journal of Wound Care.18(6): 237–8, 240–3.doi:10.12968/jowc.2009.18.6.42801.PMID19661847.
- ^abcWright A, Hawkins CH, Anggård EE, Harper DR (August 2009)."A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy".Clinical Otolaryngology.34(4): 349–357.doi:10.1111/j.1749-4486.2009.01973.x.PMID19673983.
- ^Sweere JM, Van Belleghem JD, Ishak H, Bach MS, Popescu M, Sunkari V, et al. (March 2019)."Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection".Science.363(6434): eaat9691.doi:10.1126/science.aat9691.PMC6656896.PMID30923196.
- ^Tawil N, Sacher E, Mandeville R, Meunier M (May 2012)."Surface plasmon resonance detection of E. coli and methicillin-resistant S. aureus using bacteriophages"(PDF).Biosensors & Bioelectronics.37(1): 24–29.doi:10.1016/j.bios.2012.04.048.PMID22609555.Archived(PDF)from the original on 2 February 2023.
- ^Cha K, Oh HK, Jang JY, Jo Y, Kim WK, Ha GU, et al. (10 April 2018)."Characterization of Two Novel Bacteriophages Infecting Multidrug-Resistant (MDR)Acinetobacter baumanniiand Evaluation of Their Therapeutic Efficacyin Vivo".Frontiers in Microbiology.9:696.doi:10.3389/fmicb.2018.00696.PMC5932359.PMID29755420.
- ^abSchooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, et al. (October 2017)."Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection".Antimicrobial Agents and Chemotherapy.61(10).doi:10.1128/AAC.00954-17.PMC5610518.PMID28807909.
- ^abO'Sullivan L, Bolton D, McAuliffe O, Coffey A (March 2019). "Bacteriophages in Food Applications: From Foe to Friend".Annual Review of Food Science and Technology.10(1).Annual Reviews:151–172.doi:10.1146/annurev-food-032818-121747.PMID30633564.S2CID58620015.
- ^U.S. FDA/CFSAN: Agency Response Letter, GRAS Notice No. 000198
- ^(U.S. FDA/CFSAN: Agency Response Letter, GRAS Notice No. 000218)
- ^"FSIS Directive 7120: Safe and Suitable Ingredients Used in the Production of Meat, Poultry, and Egg Products"(PDF).Food Safety and Inspection Service.Washington, DC: United States Department of Agriculture. Archived fromthe original(PDF)on 18 October 2011.
- ^Khan FM, Chen JH, Zhang R, Liu B (2023)."A comprehensive review of the applications of bacteriophage-derived endolysins for foodborne bacterial pathogens and food safety: recent advances, challenges, and future perspective".Frontiers in Microbiology.14:1259210.doi:10.3389/fmicb.2023.1259210.PMC10588457.PMID37869651.
- ^Chacón L, Barrantes K, Santamaría-Ulloa C, Solano MReyes L, Taylor LValiente C, Symonds EM, Achí R. 2020. A Somatic Coliphage Threshold Approach To Improve the Management of Activated Sludge Wastewater Treatment Plant Effluents in Resource-Limited Regions. Appl Environ Microbiol 86:e00616-20. https://doi.org/10.1128/AEM.00616-20/
- ^"FDA 510(k) Premarket Notification".U.S. Food and Drug Administration.
- ^Chacón L, Barrantes K, Santamaría-Ulloa C, Solano M, Reyes L, Taylor L, et al. (6 May 2011)."FDA clears first test to quickly diagnose and distinguish MRSA and MSSA".Applied and Environmental Microbiology.86(17). U.S. Food and Drug Administration.doi:10.1128/aem.00616-20.hdl:10669/82145.PMC7440787.PMID32591380.Archived from the original on 17 February 2015.Retrieved17 February2015.
{{cite journal}}:CS1 maint: bot: original URL status unknown (link) - ^Vaisman D (25 May 2007)."Studying anthrax in a Soviet-era lab – with Western funding".The New York Times.
- ^Dobozi-King M, Seo S, Kim JU, Young R, Cheng M, Kish LB (2005)."Rapid detection and identification of bacteria: SEnsing of Phage-Triggered Ion Cascade (SEPTIC)"(PDF).Journal of Biological Physics and Chemistry.5:3–7.doi:10.4024/1050501.jbpc.05.01.Archived fromthe original(PDF)on 26 September 2018.Retrieved19 December2016.
- ^Smith GP, Petrenko VA (April 1997). "Phage Display".Chemical Reviews.97(2): 391–410.doi:10.1021/cr960065d.PMID11848876.
- ^Liu J, Dehbi M, Moeck G, Arhin F, Bauda P, Bergeron D, et al. (February 2004). "Antimicrobial drug discovery through bacteriophage genomics".Nature Biotechnology.22(2): 185–191.doi:10.1038/nbt932.PMID14716317.S2CID9905115.
- ^"Technological background Phage-ligand technology".bioMérieux.
- ^Keen EC (January 2014)."Tradeoffs in bacteriophage life histories".Bacteriophage.4(1): e28365.doi:10.4161/bact.28365.PMC3942329.PMID24616839.
- ^Atamer Z, Samtlebe M, Neve H, J Heller K, Hinrichs J (16 July 2013)."Review: elimination of bacteriophages in whey and whey products".Frontiers in Microbiology.4:191.doi:10.3389/fmicb.2013.00191.PMC3712493.PMID23882262.
- ^Coffey A, Ross RP (August 2002). "Bacteriophage-resistance systems in dairy starter strains: molecular analysis to application".Antonie van Leeuwenhoek.82(1–4).Springer:303–321.doi:10.1023/a:1020639717181.PMID12369198.S2CID7217985.
- ^Fernández L, Escobedo S, Gutiérrez D, Portilla S, Martínez B, García P, et al. (November 2017)."Bacteriophages in the Dairy Environment: From Enemies to Allies".Antibiotics.6(4). [MDPI]: 27.doi:10.3390/antibiotics6040027.PMC5745470.PMID29117107.
- ^Abedon ST (October 2019)."Look Who's Talking: T-Even Phage Lysis Inhibition, the Granddaddy of Virus-Virus Intercellular Communication Research".Viruses.11(10): 951.doi:10.3390/v11100951.PMC6832632.PMID31623057.
- ^Mason KA, Losos JB, Singer SR, Raven PH, Johnson GB (2011).Biology.New York: McGraw-Hill. p. 533.ISBN978-0-07-893649-4.
- ^Mokrousov I (January 2009). "Corynebacterium diphtheriae: genome diversity, population structure and genotyping perspectives".Infection, Genetics and Evolution.9(1): 1–15.Bibcode:2009InfGE...9....1M.doi:10.1016/j.meegid.2008.09.011.PMID19007916.
- ^Charles RC, Ryan ET (October 2011). "Cholera in the 21st century".Current Opinion in Infectious Diseases.24(5): 472–477.doi:10.1097/QCO.0b013e32834a88af.PMID21799407.S2CID6907842.
- ^Keen EC (December 2012)."Paradigms of pathogenesis: targeting the mobile genetic elements of disease".Frontiers in Cellular and Infection Microbiology.2:161.doi:10.3389/fcimb.2012.00161.PMC3522046.PMID23248780.
- ^Drulis-Kawa Z, Majkowska-Skrobek G, Maciejewska B (2015)."Bacteriophages and phage-derived proteins--application approaches".Current Medicinal Chemistry.22(14): 1757–1773.doi:10.2174/0929867322666150209152851.PMC4468916.PMID25666799.
- ^Gabashvili IS, Khan SA, Hayes SJ, Serwer P (October 1997). "Polymorphism of bacteriophage T7".Journal of Molecular Biology.273(3): 658–667.doi:10.1006/jmbi.1997.1353.PMID9356254.
- ^Maghsoodi A, Chatterjee A, Andricioaei I, Perkins NC (December 2019)."How the phage T4 injection machinery works including energetics, forces, and dynamic pathway".Proceedings of the National Academy of Sciences of the United States of America.116(50): 25097–25105.Bibcode:2019PNAS..11625097M.doi:10.1073/pnas.1909298116.PMC6911207.PMID31767752.
- ^Fiers W, Contreras R, Duerinck F, Haegeman G, Iserentant D, Merregaert J, et al. (April 1976). "Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene".Nature.260(5551): 500–507.Bibcode:1976Natur.260..500F.doi:10.1038/260500a0.PMID1264203.S2CID4289674.
- ^Mizuno CM, Guyomar C, Roux S, Lavigne R, Rodriguez-Valera F, Sullivan MB, et al. (February 2019)."Numerous cultivated and uncultivated viruses encode ribosomal proteins".Nature Communications.10(1): 752.Bibcode:2019NatCo..10..752M.doi:10.1038/s41467-019-08672-6.PMC6375957.PMID30765709.
- ^Snustad DP (August 1968). "Dominance interactions in Escherichia coli cells mixedly infected with bacteriophage T4D wild-type and amber mutants and their possible implications as to type of gene-product function: catalytic vs. stoichiometric".Virology.35(4): 550–563.doi:10.1016/0042-6822(68)90285-7.PMID4878023.
- ^Floor E (February 1970). "Interaction of morphogenetic genes of bacteriophage T4".Journal of Molecular Biology.47(3): 293–306.doi:10.1016/0022-2836(70)90303-7.PMID4907266.
- ^Petrov AS, Harvey SC (July 2008)."Packaging double-helical DNA into viral capsids: structures, forces, and energetics".Biophysical Journal.95(2): 497–502.Bibcode:2008BpJ....95..497P.doi:10.1529/biophysj.108.131797.PMC2440449.PMID18487310.
- ^Edgar RS Conditional lethals: in Phage and the Origins of Molecular Biology (2007) Edited by John Cairns, Gunther S. Stent, and James D. Watson, Cold Spring Harbor Laboratory of Quantitative Biology, Cold Spring Harbor, Long Island, New YorkISBN978-0879698003
- ^Edgar B (October 2004)."The genome of bacteriophage T4: an archeological dig".Genetics.168(2): 575–82.doi:10.1093/genetics/168.2.575.PMC1448817.PMID15514035.
- ^abEpstein RH, Bolle A, Steinberg CM, Kellenberger E, Boy de la Tour E, Chevalley R, et al. (1963). "Physiological Studies of Conditional Lethal Mutants of Bacteriophage T4D".Cold Spring Harbor Symposia on Quantitative Biology.28:375–394.doi:10.1101/SQB.1963.028.01.053.ISSN0091-7451.
- ^Edgar RS, Lielausis I (April 1964)."Temperature-sensitive mutants of bacteriophage T4D: Their isolation and Characterization".Genetics.49(4): 649–62.doi:10.1093/genetics/49.4.649.PMC1210603.PMID14156925.
- ^Henrot C, Petit MA (November 2022)."Signals triggering prophage induction in the gut microbiota".Molecular Microbiology.118(5): 494–502.doi:10.1111/mmi.14983.PMC9827884.PMID36164818.S2CID252542284.
- ^Callaway E (2017)."Do you speak virus? Phages caught sending chemical messages".Nature.doi:10.1038/nature.2017.21313.
- ^Erez Z, Steinberger-Levy I, Shamir M, Doron S, Stokar-Avihail A, Peleg Y, et al. (January 2017)."Communication between viruses guides lysis-lysogeny decisions".Nature.541(7638): 488–493.Bibcode:2017Natur.541..488E.doi:10.1038/nature21049.PMC5378303.PMID28099413.
- ^Black LW, Thomas JA (2012). "Condensed Genome Structure".Viral Molecular Machines.Advances in Experimental Medicine and Biology. Vol. 726. Springer. pp. 469–87.doi:10.1007/978-1-4614-0980-9_21.ISBN978-1-4614-0979-3.PMC3559133.PMID22297527.
- ^Al-Shayeb B, Sachdeva R, Chen LX, Ward F, Munk P, Devoto A, et al. (February 2020)."Clades of huge phages from across Earth's ecosystems".Nature.578(7795): 425–431.Bibcode:2020Natur.578..425A.doi:10.1038/s41586-020-2007-4.PMC7162821.PMID32051592.
- ^Häuser R, Blasche S, Dokland T, Haggård-Ljungquist E, von Brunn A, Salas M, et al. (2012)."Bacteriophage protein-protein interactions".Advances in Virus Research.83:219–298.doi:10.1016/B978-0-12-394438-2.00006-2.ISBN978-0-12-394438-2.PMC3461333.PMID22748812.
- ^Morris P, Marinelli LJ, Jacobs-Sera D, Hendrix RW, Hatfull GF (March 2008)."Genomic characterization of mycobacteriophage Giles: evidence for phage acquisition of host DNA by illegitimate recombination".Journal of Bacteriology.190(6): 2172–2182.doi:10.1128/JB.01657-07.PMC2258872.PMID18178732.
- ^Krupovic M, Prangishvili D, Hendrix RW, Bamford DH (December 2011)."Genomics of bacterial and archaeal viruses: dynamics within the prokaryotic virosphere".Microbiology and Molecular Biology Reviews.75(4): 610–635.doi:10.1128/MMBR.00011-11.PMC3232739.PMID22126996.
- ^Takahashi I, Marmur J (February 1963). "Replacement of thymidylic acid by deoxyuridylic acid in the deoxyribonucleic acid of a transducing phage for Bacillus subtilis".Nature.197(4869): 794–795.Bibcode:1963Natur.197..794T.doi:10.1038/197794a0.PMID13980287.S2CID4166988.
- ^Kirnos MD, Khudyakov IY, Alexandrushkina NI, Vanyushin BF (November 1977). "2-aminoadenine is an adenine substituting for a base in S-2L cyanophage DNA".Nature.270(5635): 369–370.Bibcode:1977Natur.270..369K.doi:10.1038/270369a0.PMID413053.S2CID4177449.
- ^abKlipp E (2009).Systems biology: a textbook.Weinheim: Wiley-VCH.ISBN978-3-527-31874-2.OCLC288986435.
- ^Zhao X, Chen C, Shen W, Huang G, Le S, Lu S, et al. (January 2016)."Global Transcriptomic Analysis of Interactions between Pseudomonas aeruginosa and Bacteriophage PaP3".Scientific Reports.6:19237.Bibcode:2016NatSR...619237Z.doi:10.1038/srep19237.PMC4707531.PMID26750429.
- ^Blasche S, Wuchty S, Rajagopala SV, Uetz P (December 2013)."The protein interaction network of bacteriophage lambda with its host, Escherichia coli".Journal of Virology.87(23): 12745–12755.doi:10.1128/JVI.02495-13.PMC3838138.PMID24049175.
- ^Bobonis J, Mitosch K, Mateus A, Karcher N, Kritikos G, Selkrig J, et al. (September 2022). "Bacterial retrons encode phage-defending tripartite toxin-antitoxin systems".Nature.609(7925): 144–150.Bibcode:2022Natur.609..144B.doi:10.1038/s41586-022-05091-4.PMID35850148.S2CID250643138.
- ^Ka D, Oh H, Park E, Kim JH, Bae E (June 2020)."Structural and functional evidence of bacterial antiphage protection by Thoeris defense system via NAD+degradation ".Nature Communications.11(1): 2816.Bibcode:2020NatCo..11.2816K.doi:10.1038/s41467-020-16703-w.PMC7272460.PMID32499527.
- ^abCieślik M, Bagińska N, Jończyk-Matysiak E, Węgrzyn A, Węgrzyn G, Górski A (May 2021)."Temperate Bacteriophages-The Powerful Indirect Modulators of Eukaryotic Cells and Immune Functions".Viruses.13(6): 1013.doi:10.3390/v13061013.PMC8228536.PMID34071422.
- ^abWendling CC, Refardt D, Hall AR (February 2021)."Fitness benefits to bacteria of carrying prophages and prophage-encoded antibiotic-resistance genes peak in different environments".Evolution; International Journal of Organic Evolution.75(2): 515–528.doi:10.1111/evo.14153.PMC7986917.PMID33347602.
- ^Kirsch JM, Brzozowski RS, Faith D, Round JL, Secor PR, Duerkop BA (September 2021)."Bacteriophage-Bacteria Interactions in the Gut: From Invertebrates to Mammals".Annual Review of Virology.8(1): 95–113.doi:10.1146/annurev-virology-091919-101238.PMC8484061.PMID34255542.
- ^Brenciani A, Bacciaglia A, Vignaroli C, Pugnaloni A, Varaldo PE, Giovanetti E (January 2010)."Phim46.1, the main Streptococcus pyogenes element carrying mef(A) and tet(O) genes".Antimicrobial Agents and Chemotherapy.54(1): 221–229.doi:10.1128/AAC.00499-09.PMC2798480.PMID19858262.
- ^Jahn MT, Arkhipova K, Markert SM, Stigloher C, Lachnit T, Pita L, et al. (October 2019)."A Phage Protein Aids Bacterial Symbionts in Eukaryote Immune Evasion".Cell Host & Microbe.26(4): 542–550.e5.doi:10.1016/j.chom.2019.08.019.PMID31561965.S2CID203580138.
- ^Leigh BA (October 2019)."Cooperation among Conflict: Prophages Protect Bacteria from Phagocytosis".Cell Host & Microbe.26(4): 450–452.doi:10.1016/j.chom.2019.09.003.PMID31600498.S2CID204243652.
- ^Ali Y, Koberg S, Heßner S, Sun X, Rabe B, Back A, et al. (2014)."Temperate Streptococcus thermophilus phages expressing superinfection exclusion proteins of the Ltp type".Frontiers in Microbiology.5:98.doi:10.3389/fmicb.2014.00098.PMC3952083.PMID24659988.
- ^McGrath S, Fitzgerald GF, van Sinderen D (January 2002)."Identification and characterization of phage-resistance genes in temperate lactococcal bacteriophages".Molecular Microbiology.43(2): 509–520.doi:10.1046/j.1365-2958.2002.02763.x.PMID11985726.S2CID7084706.
- ^Douwe M, McGrath J, Fitzgerald S, van Sinderen GF.Identification and Characterization of Lactococcal-Prophage-Carried Superinfection Exclusion Genes▿ †.American Society for Microbiology (ASM).OCLC679550931.
- ^Brüssow H, Canchaya C, Hardt WD (September 2004)."Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion".Microbiology and Molecular Biology Reviews.68(3): 560–602, table of contents.doi:10.1128/MMBR.68.3.560-602.2004.PMC515249.PMID15353570.
- ^Edlin G, Lin L, Kudrna R (June 1975). "Lambda lysogens of E. coli reproduce more rapidly than non-lysogens".Nature.255(5511): 735–737.Bibcode:1975Natur.255..735E.doi:10.1038/255735a0.PMID1094307.S2CID4156346.
- ^Sekulovic O, Fortier LC (February 2015). Schaffner DW (ed.)."Global transcriptional response of Clostridium difficile carrying the CD38 prophage".Applied and Environmental Microbiology.81(4): 1364–1374.Bibcode:2015ApEnM..81.1364S.doi:10.1128/AEM.03656-14.PMC4309704.PMID25501487.
- ^Rossmann FS, Racek T, Wobser D, Puchalka J, Rabener EM, Reiger M, et al. (February 2015)."Phage-mediated dispersal of biofilm and distribution of bacterial virulence genes is induced by quorum sensing".PLOS Pathogens.11(2): e1004653.doi:10.1371/journal.ppat.1004653.PMC4338201.PMID25706310.
- ^Obeng N, Pratama AA, Elsas JD (June 2016)."The Significance of Mutualistic Phages for Bacterial Ecology and Evolution"(PDF).Trends in Microbiology.24(6): 440–449.doi:10.1016/j.tim.2015.12.009.PMID26826796.S2CID3565635.
- ^Li G, Cortez MH, Dushoff J, Weitz JS (July 2020)."When to be temperate: on the fitness benefits of lysis vs. lysogeny".Virus Evolution.6(2): veaa042.bioRxiv10.1101/709758.doi:10.1093/ve/veaa042.PMC9532926.PMID36204422.
- ^Breitbart M,Salamon P, Andresen B, Mahaffy JM, Segall AM, Mead D, et al. (October 2002)."Genomic analysis of uncultured marine viral communities".Proceedings of the National Academy of Sciences of the United States of America.99(22): 14250–14255.Bibcode:2002PNAS...9914250B.doi:10.1073/pnas.202488399.PMC137870.PMID12384570.
- ^Martin C (1988). "The Application of Bacteriophage Tracer Techniques in South West Water".Water and Environment Journal.2(6): 638–642.Bibcode:1988WaEnJ...2..638M.doi:10.1111/j.1747-6593.1988.tb01352.x.
- ^Bergh O, Børsheim KY, Bratbak G, Heldal M (August 1989). "High abundance of viruses found in aquatic environments".Nature.340(6233): 467–468.Bibcode:1989Natur.340..467B.doi:10.1038/340467a0.PMID2755508.S2CID4271861.
- ^Keen EC, Bliskovsky VV, Malagon F, Baker JD, Prince JS, Klaus JS, et al. (January 2017)."Novel" Superspreader "Bacteriophages Promote Horizontal Gene Transfer by Transformation".mBio.8(1): e02115–16.doi:10.1128/mBio.02115-16.PMC5241400.PMID28096488.
- ^Lekunberri I, Subirats J, Borrego CM, Balcázar JL (January 2017). "Exploring the contribution of bacteriophages to antibiotic resistance".Environmental Pollution.220(Pt B): 981–984.Bibcode:2017EPoll.220..981L.doi:10.1016/j.envpol.2016.11.059.hdl:10256/14115.PMID27890586.
- ^Beavogui A, Lacroix A, Wiart N, Poulain J, Delmont TO, Paoli L, et al. (March 2024)."The defensome of complex bacterial communities".Nature Communications.15(1): 2146.Bibcode:2024NatCo..15.2146B.doi:10.1038/s41467-024-46489-0.PMC10924106.PMID38459056.
- ^abManrique P, Bolduc B, Walk ST, van der Oost J, de Vos WM, Young MJ (September 2016)."Healthy human gut phageome".Proceedings of the National Academy of Sciences of the United States of America.113(37): 10400–10405.Bibcode:2016PNAS..11310400M.doi:10.1073/pnas.1601060113.PMC5027468.PMID27573828.
- ^Minot S, Sinha R, Chen J, Li H, Keilbaugh SA, Wu GD, et al. (October 2011)."The human gut virome: inter-individual variation and dynamic response to diet".Genome Research.21(10): 1616–1625.doi:10.1101/gr.122705.111.PMC3202279.PMID21880779.
- ^abcKirsch JM, Brzozowski RS, Faith D, Round JL, Secor PR, Duerkop BA (September 2021)."Bacteriophage-Bacteria Interactions in the Gut: From Invertebrates to Mammals".Annual Review of Virology.8(1): 95–113.doi:10.1146/annurev-virology-091919-101238.PMC8484061.PMID34255542.
- ^Strauss JH, Sinsheimer RL (July 1963). "Purification and properties of bacteriophage MS2 and of its ribonucleic acid".Journal of Molecular Biology.7(1): 43–54.doi:10.1016/S0022-2836(63)80017-0.PMID13978804.
- ^Miller ES, Kutter E, Mosig G, Arisaka F, Kunisawa T, Rüger W (March 2003)."Bacteriophage T4 genome".Microbiology and Molecular Biology Reviews.67(1): 86–156, table of contents.doi:10.1128/MMBR.67.1.86-156.2003.PMC150520.PMID12626685.
- ^Ackermann HW, Krisch HM (6 April 2014). "A catalogue of T4-type bacteriophages".Archives of Virology.142(12): 2329–2345.doi:10.1007/s007050050246.PMID9672598.S2CID39369249.
- ^Wang RH, Yang S, Liu Z, Zhang Y, Wang X, Xu Z, et al. (January 2024)."PhageScope: a well-annotated bacteriophage database with automatic analyses and visualizations".Nucleic Acids Research.52(D1): D756–D761.doi:10.1093/nar/gkad979.PMC10767790.PMID37904614.
Bibliography
edit- Hauser AR, Mecsas J, Moir DT (July 2016)."Beyond Antibiotics: New Therapeutic Approaches for Bacterial Infections".Clinical Infectious Diseases.63(1): 89–95.doi:10.1093/cid/ciw200.PMC4901866.PMID27025826.
- Strathdee S, Patterson T (2019).The Perfect Predator.Hachette Books.ISBN978-0-316-41808-9.
- Häusler T (2006).Viruses vs. superbugs: a solution to the antibiotics crisis?.London: Macmillan.ISBN978-1-4039-8764-8.
External links
edit- Abedon ST."The Bacteriophage Ecology Group".The Ohio State University. Archived fromthe originalon 3 June 2013.
- Tourterel C, Blouin Y."Bacteriophages illustrations and genomics".Orsay phage web site.Archived fromthe originalon 29 October 2013.Retrieved24 October2013.
- "QuipStories: Bacteriophages get a foothold on their prey"(PDF).PDBe.
- Flatow I (April 2008)."Using 'Phage' Viruses to Help Fight Infection".Science Friday podcast.NPR. Archived fromthe originalon 17 April 2008.
- "Animation of a scientifically correct T4 bacteriophage targeting E. coli bacteria".YouTube. 21 May 2019.
- "T4 Bacteriophage targetingE. colibacteria ".Animation by Hybrid Animation Medical.21 December 2009.
- Bacteriophages: What are they. Presentation by Professor Graham Hatfull, University of PittsburghonYouTube