Halobacterium(common abbreviationHbt.) is agenusin the familyHalobacteriaceae.[1]
| Halobacterium | |
|---|---|
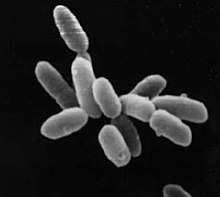
| |
| Halobacteriumsp. strain NRC-1, each cell about 5 μm in length | |
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | Halobacterium (Elazari-Volcani 1940) Elazari-Volcani 1957 non Schoop 1935
|
| Type species | |
| Halobacterium salinarum (Harrison & Kennedy 1922) Elazari-Volcani 1957
| |
| Species | |
| |
| Synonyms | |
| |
ThegenusHalobacterium( "salt" or "ocean bacterium" ) consists of several species ofArchaeawith anaerobicmetabolismwhich requires an environment with a high concentration ofsalt;many of theirproteinswill not function in low-salt environments. They grow onamino acidsin their aerobic conditions. Theircell wallsare also quite different from those ofbacteria,as ordinarylipoproteinmembranesfail in high salt concentrations. In shape, they may be either rods orcocci,and in color, either red or purple. They reproduce usingbinary fission(by constriction), and aremotile.Halobacteriumgrows best in a 42 °C environment. The genome of an unspecifiedHalobacteriumspecies, sequenced byShiladitya DasSarma,comprises 2,571,010 bp (base pairs) of DNA compiled into three circular strands: one largechromosomewith 2,014,239 bp, and two smaller ones with 191,346 and 365,425 bp. This species, calledHalobacteriumsp. NRC-1, has been extensively used for postgenomic analysis.Halobacteriumspecies can be found in theGreat Salt Lake,theDead Sea,Lake Magadi,and any other waters with high salt concentration. PurpleHalobacteriumspecies owe their color tobacteriorhodopsin,a light-sensitive protein which provides chemical energy for the cell by using sunlight to pump protons out of the cell. The resulting proton gradient across the cell membrane is used to drive the synthesis of the energy carrierATP.Thus, when these protons flow back in, they are used in the synthesis of ATP (this proton flow can be emulated with a decrease in pH outside the cell, causing a flow of H+ions). The bacteriorhodopsin protein is chemically very similar to the light-detecting pigment rhodopsin, found in thevertebrateretina.
Species ofHalobacterium
editPhylogeny
editThe currently accepted taxonomy is based on theList of Prokaryotic names with Standing in Nomenclature(LPSN)[2]andNational Center for Biotechnology Information(NCBI).[3]
| 16S rRNA basedLTP_08_2023[4][5][6] | 53 marker proteins basedGTDB08-RS214[7][8][9] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Synonyms
editSize bar = 270 nm
- Halobacterium cutirubrum>Halobacterium salinarum
- Halobacterium denitrificans>Haloferax denitrificans
- Halobacterium distributum>Halorubrum distributum
- Halobacterium halobium>Halobacterium salinarum
- Halobacterium lacusprofundi>Halorubrum lacusprofundi
- Halobacterium mediterranei>Haloferax mediterranei
- Halobacterium pharaonis>Natronomonas pharaonis
- Halobacterium piscisalsi>Halobacterium salinarum
- Halobacterium saccharovorum>Halorubrum saccharovorum
- Halobacterium sodomense>Halorubrum sodomense
- Halobacterium trapanicum>Halorubrum trapanicum
- Halobacterium vallismortis>Haloarcula vallismortis
- Halobacterium volcanii>Haloferax volcanii
Genome structure
editTheHalobacteriumNRC-1 genome is 2,571,010 bp compiled into three circularreplicons.More specifically, it is divided into one large chromosome with 2,014,239 bp and two small replicons pNRC100 (191,346 bp) and pNRC200 (365,425 bp). While much smaller than the large chromosome, the two plasmids account for most of the 91insertion sequencesand include genes for aDNA polymerase,seventranscription factors,genes in potassium and phosphate uptake, and cell division. The genome was discovered to contain a high G+C content at 67.9% on the large chromosome and 57.9% and 59.2% on the two plasmids. The genome also contained 91 insertion sequence elements constituting 12 families, including 29 on pNRC100, 40 on pNRC200, and 22 on the large chromosome. This helps explain the genetic plasticity that has been observed inHalobacterium.Of the archaea, halobacteria are viewed as being involved in the most lateral genetics (gene transfer between domains) and a proof that this transfer does take place.
Cell structure and metabolism
editHalobacteriumspecies are rod-shaped and enveloped by a single lipid bilayer membrane surrounded by anS-layermade from the cell-surface glycoprotein. They grow on amino acids in aerobic conditions. AlthoughHalobacteriumNRC-1 contains genes for glucose degradation, as well as genes for enzymes of a fatty acid oxidation pathway, it does not seem able to use these as energy sources. Though the cytoplasm retains an osmotic equilibrium with the hypersaline environment, the cell maintains a high potassium concentration using many active transporters.
ManyHalobacteriumspecies possess proteinaceous organelles called gas vesicles.
Ecology
editHalobacteria can be found in highly saline lakes such as the Great Salt Lake, the Dead Sea, and Lake Magadi.Halobacteriumcan be identified in bodies of water by the light-detecting pigment bacteriorhodopsin, which not only provides the archaeon with chemical energy, but adds to its reddish hue as well. An optimal temperature for growth has been observed at 37 °C.
Halobacteriummay be a candidate for a life form present on Mars. One of the problems associated with the survival on Mars is the destructive ultraviolet light. These microorganisms develop a thin crust of salt that can moderate some of the ultraviolet light. Sodium chloride is the most common salt and chloride salts are opaque to short-wave ultraviolet. Their photosynthetic pigment, bacteriorhodopsin, is actually opaque to the longer-wavelength ultraviolet (its red color). In addition,Halobacteriummakes pigments called bacterioruberins that are thought to protect cells from damage by ultraviolet light. The obstacle they need to overcome is being able to grow at a low temperature during a presumably short time when a pool of water could be liquid.
Applications
editFood Industry
editThere is potential forHalobacteriumspecies to be used in the food industry.[10]Some examples of uses can include the production ofBeta-Carotene,a pigment in halophilic bacteria that contributes to their red coloration, is used in the food industry as a natural food dye. Halophiles also produce degradative enzymes such aslipases,amylases,proteases,andxylanasesthat are used in various food processing methods. Notable applications of these enzymes include enhancing the fermentation process of salty foods, improving dough quality for the baking of breads, and contributing to the production of coffee.[10][11]
Bioremediation
editMany species of halophilic bacteria produceexopolysaccharides(EPS) which are used industrially asbioremediationagents.Biosurfactantsare also released by many halophilic bacteria and these amphiphilic compounds have been used for soil remediation. Many halophiles are highly tolerant of heavy metals making them potentially useful in the bioremediation ofxenobioticcompounds and heavy metals that are released into the environment from mining and metal plating. Halophiles contribute to the bioremediation of contaminants by converting xenobiotics into less toxic compounds.[11]SomeHalobacteriumspecies have been shown to be effective in the bioremediation of pollutants including aliphatic hydrocarbons, such as those found in crude oil; and aromatic hydrocarbons such as4-hydroxybenzoic acid,a contaminant in some high salinity industrial runoffs.[citation needed]
Pharmaceuticals
editSome strains ofHalobacterium,includingHalobacterium salinarum,are being explored for medical applications of their radiation-resistance mechanisms. Bacterioruberin is a carotenoid pigment found inHalobacteriumwhich decreases the bacteria’s sensitivity to γ-radiation and UV radiation.[12]
It has been shown in knockout studies, that the absence of bacterioruberin increases the sensitivity of the bacterium to oxidative DNA-damaging agents. Hydrogen peroxide, for example, reacts with bacteroruberin which prevents the production ofreactive oxygen species,and thus protects the bacterium by reducing the oxidative capacity of the DNA-damaging agent.[13]
H. salinarumalso exhibits high intracellular concentrations of potassium chloride which has also been shown to confer radiation resistance.Halobacteriumare also being explored for the pharmaceutical applications of bioactive compounds they produce, including anticancer agents, antimicrobial biosurfactancts, and antimicrobial metabolites.[12]
Significance and applications
editHalobacteria are halophilic microorganisms that are currently being studied for their uses in scientific research and biotechnology. For instance, genomic sequencing of theHalobacteriumspecies NRC-1 revealed their use of eukaryotic-like RNA polymerase II and translational machinery that are related toEscherichia coliand other Gram-negative bacteria. In addition, they possess genes for DNA replication, repair, and recombination that are similar to those present in bacteriophages, yeasts, and bacteria. The ability of thisHalobacteriumspecies to be easily cultured and genetically modified allows it to be used as amodel organismin biological studies.[14]Furthermore,HalobacteriumNRC-1 have also been employed as a potential vector for delivering vaccines. In particular, they produce gas vesicles that can be genetically engineered to display specific epitopes. Additionally, the gas vesicles demonstrate the ability to function as natural adjuvants to help evoke stronger immune responses. Because of the requirement of Halobacteria for a high-salt environment, the preparation of these gas vesicles is inexpensive and efficient, needing only tap water for their isolation.[15]
Halobacteria also contain a protein calledBacteriorhodopsinswhich are light-driven proton pumps found on the cell membrane. Although most proteins in halophiles need high salt concentrations for proper structure and functioning, this protein has shown potential to be used for biotechnological purposes because of its stability even outside of these extreme environments. Bacteriorhodopsins isolated fromHalobacterium salinarumhave been especially studied for their applications in electronics and optics. Particularly, bacteriorhodopsins have been used in holographic storage, optical switching, motion detection, andnanotechnology.Although numerous uses of this protein have been presented, there are yet to be any high-scale commercial applications established at this time.[16]
Recombination and mating
editUVirradiation ofHalobacteriumsp. strain NRC-1 induces several gene products employed inhomologous recombination.[17]For instance, ahomologof therad51/recAgene, which plays a key role in recombination, is induced 7-fold by UV. Homologous recombination may rescue stalled replication forks, and/or facilitate recombinational repair of DNA damage.[17]In its natural habitat, homologous recombination is likely induced by the UV irradiation in sunlight.
Halobacterium volcaniihas a distinctive mating system in which cytoplasmic bridges between cells appear to be used for transfer of DNA from one cell to another.[18]In wild populations ofHalorubrum,genetic exchange and recombination occur frequently.[19]This exchange may be a primitive form of sexual interaction, similar to the more well studied bacterial transformation that is also a process for transferring DNA between cells leading tohomologous recombinationalrepair of DNA damage.[citation needed]
See also
editFurther reading
editScientific journals
edit- DasSarma, S., B.R. Berquist, J.A. Coker, P. DasSarma, J.A. Müller. 2006. Post-genomics of the model haloarchaeon Halobacterium sp. NRC-1.Saline Systems 2:3.Archived2011-10-02 at theWayback Machine
- Judicial, Commission of the International Committee on Systematics of Prokaryotes (2005)."The nomenclatural types of the orders Acholeplasmatales, Halanaerobiales, Halobacteriales, Methanobacteriales, Methanococcales, Methanomicrobiales, Planctomycetales, Prochlorales, Sulfolobales, Thermococcales, Thermoproteales and Verrucomicrobiales are the genera Acholeplasma, Halanaerobium, Halobacterium, Methanobacterium, Methanococcus, Methanomicrobium, Planctomyces, Prochloron, Sulfolobus, Thermococcus, Thermoproteus and Verrucomicrobium, respectively. Opinion 79".Int. J. Syst. Evol. Microbiol.55(Pt 1): 517–518.doi:10.1099/ijs.0.63548-0.PMID15653928.
- Oren A, Ventosa A (2000)."International Committee on Systematic Bacteriology Subcommittee on the taxonomy of Halobacteriaceae. Minutes of the meetings, 16 August 1999, Sydney, Australia".Int. J. Syst. Evol. Microbiol.50(3): 1405–1407.doi:10.1099/00207713-50-3-1405.PMID10843089.
Scientific books
edit- DasSarma, S.2004. Genome sequence of an extremely halophilic archaeon, in Microbial Genomes, pp. 383–399, C.M. Fraser, T. Read, and K.E. Nelson (eds.), Humana Press, Inc., Totowa, NJ.
- Lynn Margulis,Karlene V.Schwartz,Five Kingdoms. An Illustrated Guide to the Phyla of Life on Earth(W.H.Freeman, San Francisco, 1982) pp. 36–37
- Gibbons, NE (1974). "Family V. Halobacteriaceae fam. nov.". In RE Buchanan; NE Gibbons (eds.).Bergey's Manual of Determinative Bacteriology(8th ed.). Baltimore: The Williams & Wilkins Co.
- Elazari-Volcani, B(1957). "Genus XII. Halobacterium Elazari-Volcani, 1940". In RS Breed; EGD Murray; NR Smith (eds.).Bergey's Manual of Determinative Bacteriology(7th ed.). Baltimore: The Williams & Wilkins Co. pp.207–212.
- Elazari-Volcani, B (1940). "Studies on the microflora of the Dead Sea". Doctoral dissertation, Hebrew University, Jerusalem: 1–116 and i–xiii.
{{cite journal}}:Cite journal requires|journal=(help)
References
edit- ^See theNCBIwebpage on Halobacterium.Data extracted from the"NCBI taxonomy resources".National Center for Biotechnology Information.Retrieved2007-03-19.
- ^J.P. Euzéby."Halobacterium".List of Prokaryotic names with Standing in Nomenclature(LPSN).Retrieved2021-11-17.
- ^Sayers; et al."Halobacterium".National Center for Biotechnology Information(NCBI) taxonomy database.Retrieved2022-06-05.
- ^"The LTP".Retrieved20 November2023.
- ^"LTP_all tree in newick format".Retrieved20 November2023.
- ^"LTP_08_2023 Release Notes"(PDF).Retrieved20 November2023.
- ^"GTDB release 08-RS214".Genome Taxonomy Database.Retrieved10 May2023.
- ^"ar53_r214.sp_label".Genome Taxonomy Database.Retrieved10 May2023.
- ^"Taxon History".Genome Taxonomy Database.Retrieved10 May2023.
- ^abPaterson, Russell; Lima, Nelson (2017). "Bioprospecting".Topics in Biodiversity and Convervation.Topics in Biodiversity and Conservation.16:84–91.doi:10.1007/978-3-319-47935-4.ISBN978-3-319-47933-0.
- ^abGontia-Mishra, Iti; Sapre, Swapnil; Tiwari, Sharad (August 2017)."Diversity of halophilic bacteria and actinobacteria from India and their biotechnological applications".Indian Journal of Geo-Marine Sciences.46(8): 1575–1587.Retrieved8 October2017.
- ^abJung, Kwang-Woo; Lim, Sangyong; Bahn, Yong-Sun (30 June 2017). "Microbial radiation-resistance mechanisms".Journal of Microbiology.55(7): 499–507.doi:10.1007/s12275-017-7242-5.PMID28664512.S2CID8910376.
- ^SHAHMOHAMMADI, HAMID REZA; ASGARANI, EZAT; TERATO, HIROAKI; SAITO, TAKESHI; OHYAMA, YOSHIHIKO; GEKKO, KUNIHIKO; YAMAMOTO, OSAMU; IDE, HIROSHI (1998)."Protective Roles of Bacterioruberin and Intracellular KCl in the Resistance of Halobacterium salinarium against DNA-damaging Agents".Journal of Radiation Research.39(4): 251–262.Bibcode:1998JRadR..39..251S.doi:10.1269/jrr.39.251.PMID10196780.
- ^Ng, W. V.; Kennedy, S. P.; Mahairas, G. G.; Berquist, B.; Pan, M.; Shukla, H. D.; Lasky, S. R.; Baliga, N. S.; Thorsson, V.; Sbrogna, J.; Swartzell, S.; Weir, D.; Hall, J.; Dahl, T. A.; Welti, R.; Goo, Y. A.; Leithauser, B.; Keller, K.; Cruz, R.; Danson, M. J.; Hough, D. W.; Maddocks, D. G.; Jablonski, P. E.; Krebs, M. P.; Angevine, C. M.; Dale, H.; Isenbarger, T. A.; Peck, R. F.; Pohlschroder, M.; Spudich, J. L.; Jung, K.-H.; Alam, M.; Freitas, T.; Hou, S.; Daniels, C. J.; Dennis, P. P.; Omer, A. D.; Ebhardt, H.; Lowe, T. M.; Liang, P.; Riley, M.; Hood, L.; DasSarma, S. (3 October 2000)."Genome sequence of Halobacterium species NRC-1".Proceedings of the National Academy of Sciences.97(22): 12176–12181.Bibcode:2000PNAS...9712176N.doi:10.1073/pnas.190337797.PMC17314.PMID11016950.
- ^Stuart, Elizabeth S.; Morshed, Fazeela; Sremac, Marinko; DasSarma, Shiladitya (15 June 2001). "Antigen presentation using novel particulate organelles from halophilic archaea".Journal of Biotechnology.88(2): 119–128.doi:10.1016/S0168-1656(01)00267-X.PMID11403846.
- ^Oren, Aharon (July 2010). "Industrial and environmental applications of halophilic microorganisms".Environmental Technology.31(8–9): 825–834.Bibcode:2010EnvTe..31..825O.doi:10.1080/09593330903370026.PMID20662374.S2CID16836957.
- ^abMcCready S, Müller JA, Boubriak I, Berquist BR, Ng WL, DasSarma S (2005)."UV irradiation induces homologous recombination genes in the model archaeon, Halobacterium sp. NRC-1".Saline Syst.1:3.doi:10.1186/1746-1448-1-3.PMC1224876.PMID16176594.
- ^Rosenshine I, Tchelet R, Mevarech M (1989). "The mechanism of DNA transfer in the mating system of an archaebacterium".Science.245(4924): 1387–9.Bibcode:1989Sci...245.1387R.doi:10.1126/science.2818746.PMID2818746.
- ^Papke RT, Koenig JE, Rodríguez-Valera F, Doolittle WF (2004). "Frequent recombination in a saltern population of Halorubrum".Science.306(5703): 1928–9.Bibcode:2004Sci...306.1928P.doi:10.1126/science.1103289.PMID15591201.S2CID21595153.