This articleneeds additional citations forverification.(August 2012) |
β-Hydride eliminationis a reaction in which analkylgroup bonded to a metal centre is converted into the corresponding metal-bondedhydrideand analkene.[1]The alkyl must have hydrogens on the β-carbon. For instancebutylgroups can undergo this reaction butmethylgroups cannot. The metal complex must have an empty (or vacant) sitecisto thealkylgroup for this reaction to occur. Moreover, for facile cleavage of the C–H bond, a d electron pair is needed for donation into the σ* orbital of the C–H bond. Thus, d0metals alkyls are generally more stable to β-hydride elimination than d2and higher metal alkyls and may form isolableagostic complexes,even if an empty coordination site is available.[2]
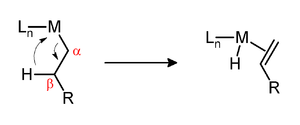
The β-hydride elimination can either be a vital step in a reaction or an unproductiveside reaction.TheShell higher olefin processrelies on β-hydride elimination to produce α-olefinswhich are used to produce detergents. Illustrative of a sometimes undesirable β-hydride elimination, β-hydride elimination inZiegler–Natta polymerizationresults in polymers of decreased molecular weight. In the case ofnickel- andpalladium-catalyzedcouplingsof aryl halides withalkyl Grignard reagents,the β-hydride elimination can lower the yield. The production of branched polymers from ethylene relies onchain walking,a key step of which is β-hydride elimination.
In some cases, β-hydride elimination is the first in a series of steps. For instance in the synthesis of RuHCl(CO)(PPh3)3fromruthenium trichloride,triphenylphosphineand2-methoxyethanol,an intermediatealkoxidecomplex undergoes a β-hydride elimination to form thehydrideligand and the pi-bondedaldehydewhich then is later converted into thecarbonyl(carbon monoxide) ligand.
Avoiding β-hydride elimination
editSeveral strategies exist for avoiding β-hydride elimination. The most common strategy is to employ alkyl ligands that do not have any hydrogen atoms at the β position. Common substituents includemethylandneopentyl.β-Hydride elimination is also inhibited when the reaction would produce a strained alkene. This situation is illustrated by the stability of metal complexes containing norbornyl ligands, where the β-hydride elimination product would violateBredt's rule.[3]
Bulky alkyl ligands, such astert-butylortrimethylsilyl,may prohibit the hydrogen atom from approaching acoplanarconfiguration with respect to the metal, and the α and β atoms. If the metal center does not have empty coordination sites, for example by the complex already having an18-electron configuration,β-hydride elimination is not possible as well.
In some cases, the coligands can impose geometries that inhibit β-hydride elimination. For the above example, the unwanted β-hydride elimination is prevented by using a diphosphine where the twophosphorusatoms are fixed apart in space. One way of doing this is to use a trans spanning ligand such asXantphos.As these metal complexes traditionally formsquare planargeometries, no vacant site cis to the alkyl group can be formed. Hence the β-hydride elimination is prevented. (Seetrans-spanning ligand.)
References
edit- ^Elschenbroich, C. (2006).Organometallics.Weinheim: Wiley-VCH.ISBN978-3-527-29390-2.
- ^Crabtree, Robert H. (2005).The organometallic chemistry of the transition metals(4th ed.). Hoboken, N.J.: John Wiley. p. 58.ISBN0-471-66256-9.OCLC61520528.
- ^Bower, Barton K.; Tennent, Howard G. (1972). "Transition metal bicyclo[2.2.1]hept-1-yls".J. Am. Chem. Soc.94:2512–2514.doi:10.1021/ja00762a056.