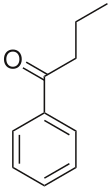
Butyrophenoneis anorganic compoundwith the formula C6H5C(O)C3H7.It is a colorless liquid.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1-Phenylbutan-1-one | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.091 | ||
PubChemCID
|
|||
| UNII | |||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H12O | |||
| Molar mass | 148.20 g/mol | ||
| Appearance | clear liquid | ||
| Melting point | 12 °C (54 °F; 285 K) | ||
| Boiling point | 229 °C (444 °F; 502 K) | ||
| poor | |||
| logP | 2.77 | ||
Refractive index(nD)
|
1.520 | ||
| Hazards | |||
| NFPA 704(fire diamond) | |||
| Flash point | 99 °C (210 °F; 372 K) | ||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
The butyrophenone structure—aketoneflanked by aphenyl ringand abutyl chain—forms the basis for many other chemicals containing varioussubstituents.Some of these butyrophenones are used to treat various psychiatric disorders such asschizophrenia,as well as acting asantiemetics.[1]
Examples of butyrophenone-derived pharmaceuticals include:
- Haloperidol,the most widely used classical antipsychotic drug in this class[1]
- Benperidol,the most potent commonly used antipsychotic (200 times more potent thanchlorpromazine)[1][2]
- Lumateperone,an atypical antipsychotic used for schizophrenia and bipolar depression
- Droperidol,Antiemeticforpostoperative nausea and vomiting
References
edit- ^abcKeith Parker; Laurence Brunton Goodman; Louis Sanford; Lazo, John S.; Gilman, Alfred (2006).Goodman & Gilman's The Pharmacological Basis of Therapeutics(11th ed.). New York: McGraw-Hill.ISBN0071422803.
- ^Grogan, Charles H.; Rice, Leonard M. (1967). "Ω-Azabicyclic Butyrophenones".Journal of Medicinal Chemistry.10(4): 621–623.doi:10.1021/jm00316a022.PMID6037051.