Carotenoids(/kəˈrɒtɪnɔɪd/) are yellow, orange, and redorganicpigmentsthat are produced byplantsandalgae,as well as several bacteria, archaea, andfungi.[1]Carotenoids give the characteristic color topumpkins,carrots,parsnips,corn,tomatoes,canaries,flamingos,salmon,lobster,shrimp,anddaffodils.Over 1,100 identified carotenoids can be further categorized into two classes –xanthophylls(which contain oxygen) andcarotenes(which are purelyhydrocarbonsand contain no oxygen).[2]
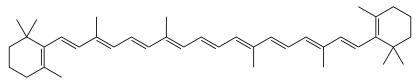
All arederivativesoftetraterpenes,meaning that they are produced from 8isopreneunits and contain 40 carbon atoms. In general, carotenoids absorb wavelengths ranging from 400 to 550 nanometers (violet to green light). This causes the compounds to be deeply colored yellow, orange, or red. Carotenoids are the dominant pigment inautumn leaf colorationof about 15-30% of tree species,[3]but many plant colors, especially reds and purples, are due topolyphenols.

Carotenoids serve two key roles in plants and algae: they absorb light energy for use inphotosynthesis,and they providephotoprotectionvianon-photochemical quenching.[4]Carotenoids that contain unsubstituted beta-ionone rings (includingβ-carotene,α-carotene,β-cryptoxanthin,andγ-carotene) havevitamin Aactivity (meaning that they can be converted toretinol). In the eye,lutein,meso-zeaxanthin,andzeaxanthinare present asmacular pigmentswhose importance in visual function, as of 2016, remains underclinical research.[3][5]
Structure and function
editCarotenoids are produced by all photosynthetic organisms and are primarily used asaccessory pigmentstochlorophyllin the light-harvesting part of photosynthesis.
They are highlyunsaturatedwithconjugated double bonds,which enables carotenoids to absorb light of variouswavelengths.At the same time, the terminal groups regulate thepolarityand properties withinlipid membranes.
Most carotenoids aretetraterpenoids,regularisoprenoids.Several modifications to these structures exist: includingcyclization,varying degrees ofsaturationor unsaturation, and otherfunctional groups.[6]Carotenes typically contain only carbon and hydrogen, i.e., they arehydrocarbons.Prominent members includeα-carotene,β-carotene,andlycopene,are known ascarotenes.Carotenoids containing oxygen includeluteinandzeaxanthin.They are known asxanthophylls.[3]Their color, ranging from pale yellow through bright orange to deep red, is directly related to their structure, especially the length of the conjugation.[3]Xanthophylls are often yellow, giving their class name.
Carotenoids also participate in different types of cell signaling.[7]They are able to signal the production ofabscisic acid,which regulates plant growth,seed dormancy,embryo maturation andgermination,cell divisionand elongation, floral growth, and stress responses.[8]
Photophysics
editThe length of the multipleconjugated double bondsdetermines their color and photophysics.[9][10]After absorbing a photon, the carotenoid transfers its excited electron tochlorophyllfor use in photosynthesis.[9]Upon absorption of light, carotenoids transfer excitation energy to and fromchlorophyll.The singlet-singlet energy transfer is a lower energy state transfer and is used during photosynthesis.[7]The triplet-triplet transfer is a higher energy state and is essential in photoprotection.[7]Light produces damaging species during photosynthesis, with the most damaging beingreactive oxygen species(ROS). As these high energy ROS are produced in the chlorophyll the energy is transferred to the carotenoid’s polyene tail and undergoes a series of reactions in which electrons are moved between the carotenoid bonds in order to find the most balanced (lowest energy) state for the carotenoid.[9]
Carotenoids defend plants againstsinglet oxygen,by both energy transfer and by chemical reactions. They also protect plants by quenching triplet chlorophyll.[11]By protecting lipids from free-radical damage, which generate chargedlipid peroxidesand other oxidised derivatives, carotenoids support crystalline architecture and hydrophobicity of lipoproteins and cellular lipid structures, hence oxygen solubility and its diffusion therein.[12]
Structure-property relationships
editLike somefatty acids,carotenoids arelipophilicdue to the presence of longunsaturatedaliphaticchains.[3]As a consequence, carotenoids are typically present in plasmalipoproteinsand cellular lipid structures.[13]
Morphology
editCarotenoids are located primarily outside thecell nucleusin different cytoplasm organelles,lipid droplets,cytosomesand granules. They have been visualised and quantified byraman spectroscopyin analgalcell.[14]
With the development ofmonoclonal antibodiestotrans-lycopeneit was possible to localise this carotenoid in different animal and human cells.[15]
Foods
editBeta-carotene,found inpumpkins,sweet potato,carrotsandwinter squash,is responsible for their orange-yellow colors.[3]Dried carrots have the highest amount of carotene of any food per 100-gram serving, measured in retinol activity equivalents (provitamin A equivalents).[3][16]Vietnamesegacfruit contains the highest known concentration of the carotenoidlycopene.[17]Although green,kale,spinach,collard greens,andturnip greenscontain substantial amounts of beta-carotene.[3]The diet offlamingosis rich in carotenoids, imparting the orange-colored feathers of these birds.[18]
Reviews of preliminary research in 2015 indicated that foods high in carotenoids may reduce the risk ofhead and neck cancers[19]andprostate cancer.[20]There is no correlation between consumption of foods high in carotenoids and vitamin A and the risk ofParkinson's disease.[21]
Humans and otheranimalsare mostly incapable of synthesizing carotenoids, and must obtain them through their diet. Carotenoids are a common and often ornamental feature in animals. For example, the pink color ofsalmon,and the red coloring of cookedlobstersand scales of the yellow morph ofcommon wall lizardsare due to carotenoids.[22][citation needed]It has been proposed that carotenoids are used in ornamental traits (for extreme examples seepuffinbirds) because, given their physiological and chemical properties, they can be used as visible indicators of individual health, and hence are used by animals when selecting potential mates.[23]
Carotenoids from the diet are stored in the fatty tissues of animals,[3]and exclusivelycarnivorousanimals obtain the compounds from animal fat. In the human diet,absorptionof carotenoids is improved when consumed with fat in a meal.[24]Cooking carotenoid-containing vegetables in oil and shredding the vegetable both increase carotenoidbioavailability.[3][24][25]
Plant colors
editThe most common carotenoids include lycopene and the vitamin A precursor β-carotene. In plants, the xanthophyllluteinis the most abundant carotenoid and its role in preventing age-related eye disease is currently under investigation.[5]Lutein and the other carotenoid pigments found in mature leaves are often not obvious because of the masking presence ofchlorophyll.When chlorophyll is not present, as in autumn foliage, the yellows and oranges of the carotenoids are predominant. For the same reason, carotenoid colors often predominate in ripe fruit after being unmasked by the disappearance of chlorophyll.
Carotenoids are responsible for the brilliant yellows and oranges that tintdeciduousfoliage (such as dyingautumn leaves) of certain hardwood species ashickories,ash,maple,yellow poplar,aspen,birch,black cherry,sycamore,cottonwood,sassafras,andalder.Carotenoids are the dominant pigment in autumn leaf coloration of about 15-30% of tree species.[26]However, the reds, the purples, and their blended combinations that decorate autumn foliage usually come from another group of pigments in the cells calledanthocyanins.Unlike the carotenoids, these pigments are not present in the leaf throughout the growing season, but are actively produced towards the end of summer.[27]
Bird colors and sexual selection
editDietary carotenoids and their metabolic derivatives are responsible for bright yellow to red coloration in birds.[28]Studies estimate that around 2956 modern bird species display carotenoid coloration and that the ability to utilize these pigments for external coloration has evolved independently many times throughout avian evolutionary history.[29]Carotenoid coloration exhibits high levels ofsexual dimorphism,with adult male birds generally displaying more vibrant coloration than females of the same species.[30]
These differences arise due to the selection of yellow and red coloration in males byfemale preference.[31][30]In many species of birds, females invest greater time and resources into raising offspring than their male partners. Therefore, it is imperative that female birds carefully select high quality mates. Current literature supports the theory that vibrant carotenoid coloration is correlated with male quality—either though direct effects on immune function and oxidative stress,[32][33][34]or through a connection between carotenoid metabolizing pathways and pathways for cellular respiration.[35][36]
It is generally considered that sexually selected traits, such as carotenoid-based coloration, evolve because they are honest signals of phenotypic and genetic quality. For instance, among males of the bird speciesParus major,the more colorfully ornamented males produce sperm that is better protected againstoxidative stressdue to increased presence of carotenoidantioxidants.[37]However, there is also evidence that attractive male coloration may be a faulty signal of male quality. Amongsticklebackfish, males that are more attractive to females due to carotenoid colorants appear to under-allocate carotenoids to their germline cells.[38]Since carotinoids are beneficial antioxidants, their under-allocation togermlinecells can lead to increased oxidativeDNA damageto these cells.[38]Therefore, female sticklebacks may riskfertilityand the viability of their offspring by choosing redder, but more deteriorated partners with reducedspermquality.
Aroma chemicals
editProducts of carotenoid degradation such asionones,damasconesanddamascenonesare also important fragrance chemicals that are used extensively in theperfumesand fragrance industry. Both β-damascenone and β-ionone although low in concentration inrosedistillates are the key odor-contributing compounds in flowers. In fact, the sweet floral smells present inblack tea,agedtobacco,grape,and manyfruitsare due to the aromatic compounds resulting from carotenoid breakdown.
Disease
editSome carotenoids are produced by bacteria to protect themselves from oxidative immune attack. Theaureus(golden) pigment that gives some strains ofStaphylococcus aureustheir name is a carotenoid calledstaphyloxanthin.This carotenoid is a virulence factor with anantioxidantaction that helps the microbe evade death byreactive oxygen speciesused by the host immune system.[39]
Biosynthesis
editThe basic building blocks of carotenoids areisopentenyl diphosphate(IPP) anddimethylallyl diphosphate(DMAPP).[40]These two isoprene isomers are used to create various compounds depending on the biological pathway used to synthesize the isomers.[41]Plants are known to use two different pathways for IPP production: the cytosolicmevalonic acidpathway (MVA) and the plastidicmethylerythritol 4-phosphate(MEP).[40]In animals, the production ofcholesterolstarts by creating IPP and DMAPP using the MVA.[41]For carotenoid production plants use MEP to generate IPP and DMAPP.[40]The MEP pathway results in a 5:1 mixture of IPP:DMAPP.[41]IPP and DMAPP undergo several reactions, resulting in the major carotenoid precursor,geranylgeranyl diphosphate(GGPP). GGPP can be converted into carotenes or xanthophylls by undergoing a number of different steps within the carotenoid biosynthetic pathway.[40]
MEP pathway
editGlyceraldehyde 3-phosphateandpyruvate,intermediates ofphotosynthesis,are converted to deoxy-D-xylulose 5-phosphate (DXP) catalyzed byDXP synthase(DXS).DXP reductoisomerasecatalyzes the reduction byNADPHand subsequent rearrangement.[40][41]The resulting MEP is converted to 4-(cytidine 5’-diphospho)-2-C-methyl-D-erythritol (CDP-ME) in the presence of CTP using the enzyme MEP cytidylyltransferase. CDP-ME is then converted, in the presence ofATP,to 2-phospho-4-(cytidine 5’-diphospho)-2-C-methyl-D-erythritol (CDP-ME2P). The conversion to CDP-ME2P is catalyzed byCDP-ME kinase.Next, CDP-ME2P is converted to 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MECDP). This reaction occurs when MECDP synthase catalyzes the reaction and CMP is eliminated from the CDP-ME2P molecule. MECDP is then converted to (e)-4-hydroxy-3-methylbut-2-en-1-yl diphosphate (HMBDP) viaHMBDP synthasein the presence offlavodoxinand NADPH. HMBDP is reduced to IPP in the presence offerredoxinand NADPH by the enzymeHMBDP reductase.The last two steps involving HMBPD synthase and reductase can only occur in completelyanaerobicenvironments. IPP is then able toisomerizeto DMAPP via IPP isomerase.[41]
Carotenoid biosynthetic pathway
editTwo GGPP molecules condense viaphytoene synthase(PSY), forming the 15-cisisomerofphytoene.PSY belongs to thesqualene/phytoene synthase familyand is homologous tosqualene synthasethat takes part insteroidbiosynthesis. The subsequent conversion of phytoene into all-trans-lycopenedepends on the organism. Bacteria and fungi employ a single enzyme, thebacterial phytoene desaturase(CRTI) for the catalysis. Plants and cyanobacteria however utilize four enzymes for this process.[42]The first of these enzymes is aplant-type phytoene desaturasewhich introduces two additional double bonds into 15-cis-phytoene bydehydrogenationand isomerizes two of its existing double bonds fromtrans to cisproducing 9,15,9’-tri-cis-ζ-carotene. The central double bond of this tri-cis-ζ-carotene is isomerized by thezeta-carotene isomeraseZ-ISO and the resulting 9,9'-di-cis-ζ-carotene is dehydrogenated again via aζ-carotene desaturase (ZDS).This again introduces two double bonds, resulting in 7,9,7’,9’-tetra-cis-lycopene.CRTISO,a carotenoid isomerase, is needed to convert thecis-lycopene into anall-translycopene in the presence of reducedFAD.
This all-trans lycopene is cyclized;cyclizationgives rise to carotenoid diversity, which can be distinguished based on the end groups. There can be either abeta ringor an epsilon ring, each generated by a different enzyme (lycopene beta-cyclase[beta-LCY] orlycopene epsilon-cyclase[epsilon-LCY]).α-Caroteneis produced when the all-trans lycopene first undergoes reaction with epsilon-LCY then a second reaction with beta-LCY; whereasβ-caroteneis produced by two reactions with beta-LCY. α- and β-Carotene are the most common carotenoids in the plantphotosystemsbut they can still be further converted into xanthophylls by using beta-hydrolase and epsilon-hydrolase, leading to a variety of xanthophylls.[40]
Regulation
editIt is believed that both DXS and DXR are rate-determining enzymes, allowing them to regulate carotenoid levels.[40]This was discovered in an experiment where DXS and DXR were genetically overexpressed, leading to increased carotenoid expression in the resulting seedlings.[40]Also, J-protein (J20) and heat shock protein 70 (Hsp70) chaperones are thought to be involved in post-transcriptional regulation of DXS activity, such that mutants with defective J20 activity exhibit reduced DXS enzyme activity while accumulating inactive DXS protein.[43]Regulation may also be caused by externaltoxinsthat affect enzymes and proteins required for synthesis. Ketoclomazone is derived fromherbicidesapplied to soil and binds to DXP synthase.[41]This inhibits DXP synthase, preventing synthesis of DXP and halting the MEP pathway.[41]The use of this toxin leads to lower levels of carotenoids in plants grown in the contaminated soil.[41]Fosmidomycin,anantibiotic,is acompetitive inhibitorof DXP reductoisomerase due to its similar structure to the enzyme.[41]Application of said antibiotic prevents reduction of DXP, again halting the MEP pathway.[41]
Naturally occurring carotenoids
edit- Hydrocarbons
- Lycopersene7,8,11,12,15,7',8',11',12',15'-Decahydro-γ,γ-carotene
- Phytofluene
- Lycopene
- Hexahydrolycopene15-cis-7,8,11,12,7',8'-Hexahydro-γ,γ-carotene
- Torulene3',4'-Didehydro-β,γ-carotene
- α-Zeacarotene7',8'-Dihydro-ε,γ-carotene
- α-Carotene
- β-Carotene
- γ-Carotene
- δ-Carotene
- ε-Carotene
- ζ-Carotene
- Alcohols
- Alloxanthin
- Bacterioruberin2,2'-Bis(3-hydroxy-3-methylbutyl)-3,4,3',4'-tetradehydro-1,2,1',2'-tetrahydro-γ,γ-carotene-1,1'-diol
- Cynthiaxanthin
- Pectenoxanthin
- Cryptomonaxanthin(3R,3'R)-7,8,7',8'-Tetradehydro-β,β-carotene-3,3'-diol
- Crustaxanthinβ,-Carotene-3,4,3',4'-tetrol
- Gazaniaxanthin(3R)-5'-cis-β,γ-Caroten-3-ol
- OH-Chlorobactene1',2'-Dihydro-f,γ-caroten-1'-ol
- Loroxanthinβ,ε-Carotene-3,19,3'-triol
- Lutein(3R,3′R,6′R)-β,ε-carotene-3,3′-diol
- Lycoxanthinγ,γ-Caroten-16-ol
- Rhodopin1,2-Dihydro-γ,γ-caroten-l-ol
- Rhodopinola.k.a.Warmingol13-cis-1,2-Dihydro-γ,γ-carotene-1,20-diol
- Saproxanthin3',4'-Didehydro-1',2'-dihydro-β,γ-carotene-3,1'-diol
- Zeaxanthin
- Glycosides
- Oscillaxanthin2,2'-Bis(β-L-rhamnopyranosyloxy)-3,4,3',4'-tetradehydro-1,2,1',2'-tetrahydro-γ,γ-carotene-1,1'-diol
- Phleixanthophyll1'-(β-D-Glucopyranosyloxy)-3',4'-didehydro-1',2'-dihydro-β,γ-caroten-2'-ol
- Ethers
- Rhodovibrin1'-Methoxy-3',4'-didehydro-1,2,1',2'-tetrahydro-γ,γ-caroten-1-ol
- Spheroidene1-Methoxy-3,4-didehydro-1,2,7',8'-tetrahydro-γ,γ-carotene
- Epoxides
- Diadinoxanthin5,6-Epoxy-7',8'-didehydro-5,6-dihydro—carotene-3,3-diol
- Luteoxanthin5,6: 5',8'-Diepoxy-5,6,5',8'-tetrahydro-β,β-carotene-3,3'-diol
- Mutatoxanthin
- Citroxanthin
- Zeaxanthin furanoxide5,8-Epoxy-5,8-dihydro-β,β-carotene-3,3'-diol
- Neochrome5',8'-Epoxy-6,7-didehydro-5,6,5',8'-tetrahydro-β,β-carotene-3,5,3'-triol
- Foliachrome
- Trollichrome
- Vaucheriaxanthin5',6'-Epoxy-6,7-didehydro-5,6,5',6'-tetrahydro-β,β-carotene-3,5,19,3'-tetrol
- Aldehydes
- Rhodopinal
- Warmingone13-cis-1-Hydroxy-1,2-dihydro-γ,γ-caroten-20-al
- Torularhodinaldehyde3',4'-Didehydro-β,γ-caroten-16'-al
- Acids and acid esters
- Torularhodin3',4'-Didehydro-β,γ-caroten-16'-oic acid
- Torularhodin methyl esterMethyl 3',4'-didehydro-β,γ-caroten-16'-oate
- Ketones
- Astacene
- Astaxanthin
- Canthaxanthin[44]a.k.a. Aphanicin, Chlorellaxanthin β,β-Carotene-4,4'-dione
- Capsanthin(3R,3'S,5'R)-3,3'-Dihydroxy-β,κ-caroten-6'-one
- Capsorubin(3S,5R,3'S,5'R)-3,3'-Dihydroxy-κ,κ-carotene-6,6'-dione
- Cryptocapsin(3'R,5'R)-3'-Hydroxy-β,κ-caroten-6'-one
- 2,2'-Diketospirilloxanthin1,1'-Dimethoxy-3,4,3',4'-tetradehydro-1,2,1',2'-tetrahydro-γ,γ-carotene-2,2'-dione
- Echinenoneβ,β-Caroten-4-one
- 3'-Hydroxyechinenone
- Flexixanthin3,1'-Dihydroxy-3',4'-didehydro-1',2'-dihydro-β,γ-caroten-4-one
- 3-OH-Canthaxanthin a.k.a. Adonirubin a.k.a.Phoenicoxanthin3-Hydroxy-β,β-carotene-4,4'-dione
- Hydroxyspheriodenone1'-Hydroxy-1-methoxy-3,4-didehydro-1,2,1',2',7',8'-hexahydro-γ,γ-caroten-2-one
- Okenone1'-Methoxy-1',2'-dihydro-c,γ-caroten-4'-one
- Pectenolone3,3'-Dihydroxy-7',8'-didehydro-β,β-caroten-4-one
- Phoenicononea.k.a.Dehydroadonirubin3-Hydroxy-2,3-didehydro-β,β-carotene-4,4'-dione
- Phoenicopteroneβ,ε-caroten-4-one
- Rubixanthone3-Hydroxy-β,γ-caroten-4'-one
- Siphonaxanthin3,19,3'-Trihydroxy-7,8-dihydro-β,ε-caroten-8-one
- Esters of alcohols
- Astacein3,3'-Bispalmitoyloxy-2,3,2',3'-tetradehydro-β,β-carotene-4,4'-dione or 3,3'-dihydroxy-2,3,2',3'-tetradehydro-β,β-carotene-4,4'-dione dipalmitate
- Fucoxanthin3'-Acetoxy-5,6-epoxy-3,5'-dihydroxy-6',7'-didehydro-5,6,7,8,5',6'-hexahydro-β,β-caroten-8-one
- Isofucoxanthin3'-Acetoxy-3,5,5'-trihydroxy-6',7'-didehydro-5,8,5',6'-tetrahydro-β,β-caroten-8-one
- Physalien
- Siphonein3,3'-Dihydroxy-19-lauroyloxy-7,8-dihydro-β,ε-caroten-8-one or 3,19,3'-trihydroxy-7,8-dihydro-β,ε-caroten-8-one 19-laurate
- Apocarotenoids
- β-Apo-2'-carotenal3',4'-Didehydro-2'-apo-b-caroten-2'-al
- Apo-2-lycopenal
- Apo-6'-lycopenal6'-Apo-y-caroten-6'-al
- Azafrinaldehyde5,6-Dihydroxy-5,6-dihydro-10'-apo-β-caroten-10'-al
- Bixin6'-Methyl hydrogen 9'-cis-6,6'-diapocarotene-6,6'-dioate
- Citranaxanthin5',6'-Dihydro-5'-apo-β-caroten-6'-one or 5',6'-dihydro-5'-apo-18'-nor-β-caroten-6'-one or 6'-methyl-6'-apo-β-caroten-6'-one
- Crocetin8,8'-Diapo-8,8'-carotenedioic acid
- Crocetinsemialdehyde8'-Oxo-8,8'-diapo-8-carotenoic acid
- CrocinDigentiobiosyl 8,8'-diapo-8,8'-carotenedioate
- Hopkinsiaxanthin3-Hydroxy-7,8-didehydro-7',8'-dihydro-7'-apo-b-carotene-4,8'-dione or 3-hydroxy-8'-methyl-7,8-didehydro-8'-apo-b-carotene-4,8'-dione
- Methyl apo-6'-lycopenoateMethyl 6'-apo-y-caroten-6'-oate
- Paracentrone3,5-Dihydroxy-6,7-didehydro-5,6,7',8'-tetrahydro-7'-apo-b-caroten-8'-one or 3,5-dihydroxy-8'-methyl-6,7-didehydro-5,6-dihydro-8'-apo-b-caroten-8'-one
- Sintaxanthin7',8'-Dihydro-7'-apo-b-caroten-8'-one or 8'-methyl-8'-apo-b-caroten-8'-one
- Nor- and seco-carotenoids
- Actinioerythrin3,3'-Bisacyloxy-2,2'-dinor-b,b-carotene-4,4'-dione
- β-Carotenone5,6:5',6'-Diseco-b,b-carotene-5,6,5',6'-tetrone
- Peridinin3'-Acetoxy-5,6-epoxy-3,5'-dihydroxy-6',7'-didehydro-5,6,5',6'-tetrahydro-12',13',20'-trinor-b,b-caroten-19,11-olide
- Pyrrhoxanthininol5,6-epoxy-3,3'-dihydroxy-7',8'-didehydro-5,6-dihydro-12',13',20'-trinor-b,b-caroten-19,11-olide
- Semi-α-carotenone5,6-Seco-b,e-carotene-5,6-dione
- Semi-β-carotenone5,6-seco-b,b-carotene-5,6-dione or 5',6'-seco-b,b-carotene-5',6'-dione
- Triphasiaxanthin3-Hydroxysemi-b-carotenone 3'-Hydroxy-5,6-seco-b,b-carotene-5,6-dione or 3-hydroxy-5',6'-seco-b,b-carotene-5',6'-dione
- Retro-carotenoids and retro-apo-carotenoids
- Eschscholtzxanthin4',5'-Didehydro-4,5'-retro-b,b-carotene-3,3'-diol
- Eschscholtzxanthone3'-Hydroxy-4',5'-didehydro-4,5'-retro-b,b-caroten-3-one
- Rhodoxanthin4',5'-Didehydro-4,5'-retro-b,b-carotene-3,3'-dione
- Tangeraxanthin3-Hydroxy-5'-methyl-4,5'-retro-5'-apo-b-caroten-5'-one or 3-hydroxy-4,5'-retro-5'-apo-b-caroten-5'-one
- Higher carotenoids
- Nonaprenoxanthin2-(4-Hydroxy-3-methyl-2-butenyl)-7',8',11',12'-tetrahydro-e,y-carotene
- Decaprenoxanthin2,2'-Bis(4-hydroxy-3-methyl-2-butenyl)-e,e-carotene
- C.p. 4502-[4-Hydroxy-3-(hydroxymethyl)-2-butenyl]-2'-(3-methyl-2-butenyl)-b,b-carotene
- C.p. 4732'-(4-Hydroxy-3-methyl-2-butenyl)-2-(3-methyl-2-butenyl)-3',4'-didehydro-l',2'-dihydro-β,γ-caroten-1'-ol
- Bacterioruberin2,2'-Bis(3-hydroxy-3-methylbutyl)-3,4,3',4'-tetradehydro-1,2,1',2'-tetrahydro-γ,γ-carotene-1,1'-diol
See also
edit- List of phytochemicals in food
- CRT (genetics),gene clusterresponsible for thebiosynthesisof carotenoids
- E number#E100–E199 (colours)
- Phytochemistry
References
edit- ^Nelson, David L.; Cox, Michael M. (2005).Principles of Biochemistry(4th ed.). New York: W. H. Freeman.ISBN0-7167-4339-6.
- ^Yabuzaki, Junko (2017-01-01)."Carotenoids Database: structures, chemical fingerprints and distribution among organisms".Database.2017(1).doi:10.1093/database/bax004.PMC5574413.PMID28365725.
- ^abcdefghij"Carotenoids".Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 1 August 2016.Retrieved17 April2019.
- ^Armstrong GA, Hearst JE (1996)."Carotenoids 2: Genetics and molecular biology of carotenoid pigment biosynthesis".FASEB J.10(2): 228–37.doi:10.1096/fasebj.10.2.8641556.PMID8641556.S2CID22385652.
- ^abBernstein, P. S.; Li, B; Vachali, P. P.; Gorusupudi, A; Shyam, R; Henriksen, B. S.; Nolan, J. M. (2015)."Lutein, Zeaxanthin, and meso-Zeaxanthin: The Basic and Clinical Science Underlying Carotenoid-based Nutritional Interventions against Ocular Disease".Progress in Retinal and Eye Research.50:34–66.doi:10.1016/j.preteyeres.2015.10.003.PMC4698241.PMID26541886.
- ^Maresca, Julia A.; Romberger, Steven P.; Bryant, Donald A. (2008-05-28)."Isorenieratene Biosynthesis in Green Sulfur Bacteria Requires the Cooperative Actions of Two Carotenoid Cyclases".Journal of Bacteriology.190(19): 6384–6391.doi:10.1128/JB.00758-08.ISSN0021-9193.PMC2565998.PMID18676669.
- ^abcCogdell, R. J. (1978-11-30). "Carotenoids in photosynthesis".Phil. Trans. R. Soc. Lond. B.284(1002): 569–579.Bibcode:1978RSPTB.284..569C.doi:10.1098/rstb.1978.0090.ISSN0080-4622.
- ^Finkelstein, Ruth (2013-11-01)."Abscisic Acid Synthesis and Response".The Arabidopsis Book.11:e0166.doi:10.1199/tab.0166.ISSN1543-8120.PMC3833200.PMID24273463.
- ^abcVershinin, Alexander (1999-01-01). "Biological functions of carotenoids - diversity and evolution".BioFactors.10(2–3): 99–104.doi:10.1002/biof.5520100203.ISSN1872-8081.PMID10609869.S2CID24408277.
- ^Polívka, Tomáš; Sundström, Villy (2004). "Ultrafast Dynamics of Carotenoid Excited States−From Solution to Natural and Artificial Systems".Chemical Reviews.104(4): 2021–2072.doi:10.1021/cr020674n.PMID15080720.
- ^Ramel, Fanny; Birtic, Simona; Cuiné, Stéphan; Triantaphylidès, Christian; Ravanat, Jean-Luc; Havaux, Michel (2012)."Chemical Quenching of Singlet Oxygen by Carotenoids in Plants".Plant Physiology.158(3): 1267–1278.doi:10.1104/pp.111.182394.PMC3291260.PMID22234998.
- ^John Thomas Landrum (2010).Carotenoids: physical, chemical, and biological functions and properties.Boca Raton: CRC Press.ISBN978-1-4200-5230-5.OCLC148650411.
- ^Gruszecki, Wieslaw I. (2004), Frank, Harry A.; Young, Andrew J.; Britton, George; Cogdell, Richard J. (eds.),"Carotenoids in Membranes",The Photochemistry of Carotenoids,Advances in Photosynthesis and Respiration, vol. 8, Dordrecht: Kluwer Academic Publishers, pp. 363–379,doi:10.1007/0-306-48209-6_20,ISBN978-0-7923-5942-5,retrieved2021-03-28
- ^Timlin, Jerilyn A.; Collins, Aaron M.; Beechem, Thomas A.; Shumskaya, Maria; Wurtzel, Eleanore T. (2017-06-14), "Localizing and Quantifying Carotenoids in Intact Cells and Tissues",Carotenoids,InTech,doi:10.5772/68101,ISBN978-953-51-3211-0,S2CID54807067
- ^Petyaev, Ivan M.; Zigangirova, Naylia A.; Pristensky, Dmitry; et al. (2018)."Non-Invasive Immunofluorescence Assessment of Lycopene Supplementation Status in Skin Smears".Monoclonal Antibodies in Immunodiagnosis and Immunotherapy.37(3): 139–146.doi:10.1089/mab.2018.0012.ISSN2167-9436.PMID29901405.S2CID49190846.
- ^"Foods Highest in Retinol Activity Equivalent".nutritiondata.self.com.Retrieved2015-12-04.
- ^Tran, X. T.; Parks, S. E.; Roach, P. D.; Golding, J. B.; Nguyen, M. H. (2015)."Effects of maturity on physicochemical properties of Gac fruit (Momordica cochinchinensis Spreng.)".Food Science & Nutrition.4(2): 305–314.doi:10.1002/fsn3.291.PMC4779482.PMID27004120.
- ^Yim, K. J.; Kwon, J; Cha, I. T.; Oh, K. S.; Song, H. S.; Lee, H. W.; Rhee, J. K.; Song, E. J.; Rho, J. R.; Seo, M. L.; Choi, J. S.; Choi, H. J.; Lee, S. J.; Nam, Y. D.; Roh, S. W. (2015)."Occurrence of viable, red-pigmented haloarchaea in the plumage of captive flamingoes".Scientific Reports.5:16425.Bibcode:2015NatSR...516425Y.doi:10.1038/srep16425.PMC4639753.PMID26553382.
- ^Leoncini; Sources, Natural; Head; Cancer, Neck; et al. (July 2015). "A Systematic Review and Meta-analysis of Epidemiological Studies".Cancer Epidemiol Biomarkers Prev.24(7): 1003–11.doi:10.1158/1055-9965.EPI-15-0053.PMID25873578.S2CID21131127.
- ^Soares Nda, C; et al. (October 2015)."Anticancer properties of carotenoids in prostate cancer. A review"(PDF).Histol Histopathol.30(10): 1143–54.doi:10.14670/HH-11-635.PMID26058846.
- ^Takeda, A; et al. (2014)."Vitamin A and carotenoids and the risk of Parkinson's disease: a systematic review and meta-analysis".Neuroepidemiology.42(1): 25–38.doi:10.1159/000355849.PMID24356061.S2CID12396064.
- ^Sacchi, Roberto (4 June 2013). "Colour variation in the polymorphic common wall lizard (Podarcis muralis): An analysis using the RGB colour system".Zoologischer Anzeiger.252(4): 431–439.doi:10.1016/j.jcz.2013.03.001.
- ^Whitehead RD, Ozakinci G, Perrett DI (2012)."Attractive skin coloration: harnessing sexual selection to improve diet and health".Evol Psychol.10(5): 842–54.doi:10.1177/147470491201000507.PMC10429994.PMID23253790.S2CID8655801.
- ^abMashurabad, Purna Chandra; Palika, Ravindranadh; Jyrwa, Yvette Wilda; Bhaskarachary, K.; Pullakhandam, Raghu (3 January 2017)."Dietary fat composition, food matrix and relative polarity modulate the micellarization and intestinal uptake of carotenoids from vegetables and fruits".Journal of Food Science and Technology.54(2): 333–341.doi:10.1007/s13197-016-2466-7.ISSN0022-1155.PMC5306026.PMID28242932.
- ^Rodrigo, María Jesús; Cilla, Antonio; Barberá, Reyes; Zacarías, Lorenzo (2015). "Carotenoid bioaccessibility in pulp and fresh juice from carotenoid-rich sweet oranges and mandarins".Food & Function.6(6): 1950–1959.doi:10.1039/c5fo00258c.PMID25996796.
- ^Archetti, Marco; Döring, Thomas F.; Hagen, Snorre B.; Hughes, Nicole M.; Leather, Simon R.; Lee, David W.; Lev-Yadun, Simcha; Manetas, Yiannis; Ougham, Helen J. (2011). "Unravelling the evolution of autumn colours: an interdisciplinary approach".Trends in Ecology & Evolution.24(3): 166–73.doi:10.1016/j.tree.2008.10.006.PMID19178979.
- ^Davies, Kevin M., ed. (2004).Plant pigments and their manipulation.Annual Plant Reviews. Vol. 14. Oxford: Blackwell Publishing. p. 6.ISBN978-1-4051-1737-1.
- ^Delhey, Kaspar; Peters, Anne (2016-11-16)."The effect of colour-producing mechanisms on plumage sexual dichromatism in passerines and parrots".Functional Ecology.31(4): 903–914.doi:10.1111/1365-2435.12796.ISSN0269-8463.
- ^Thomas, Daniel B.; McGraw, Kevin J.; Butler, Michael W.; Carrano, Matthew T.; Madden, Odile; James, Helen F. (2014-08-07)."Ancient origins and multiple appearances of carotenoid-pigmented feathers in birds".Proceedings of the Royal Society B: Biological Sciences.281(1788): 20140806.doi:10.1098/rspb.2014.0806.ISSN0962-8452.PMC4083795.PMID24966316.
- ^abCooney, Christopher R.; Varley, Zoë K.; Nouri, Lara O.; Moody, Christopher J. A.; Jardine, Michael D.; Thomas, Gavin H. (2019-04-16)."Sexual selection predicts the rate and direction of colour divergence in a large avian radiation".Nature Communications.10(1): 1773.Bibcode:2019NatCo..10.1773C.doi:10.1038/s41467-019-09859-7.ISSN2041-1723.PMC6467902.PMID30992444.
- ^Hill, Geoffrey E. (September 1990)."Female house finches prefer colourful males: sexual selection for a condition-dependent trait".Animal Behaviour.40(3): 563–572.doi:10.1016/s0003-3472(05)80537-8.ISSN0003-3472.S2CID53176725.
- ^Weaver, Ryan J.; Santos, Eduardo S. A.; Tucker, Anna M.; Wilson, Alan E.; Hill, Geoffrey E. (2018-01-08)."Carotenoid metabolism strengthens the link between feather coloration and individual quality".Nature Communications.9(1): 73.Bibcode:2018NatCo...9...73W.doi:10.1038/s41467-017-02649-z.ISSN2041-1723.PMC5758789.PMID29311592.
- ^Simons, Mirre J. P.; Cohen, Alan A.; Verhulst, Simon (2012-08-14)."What Does Carotenoid-Dependent Coloration Tell? Plasma Carotenoid Level Signals Immunocompetence and Oxidative Stress State in Birds–A Meta-Analysis".PLOS ONE.7(8): e43088.Bibcode:2012PLoSO...743088S.doi:10.1371/journal.pone.0043088.ISSN1932-6203.PMC3419220.PMID22905205.
- ^Koch, Rebecca E.; Hill, Geoffrey E. (2018-05-14)."Do carotenoid-based ornaments entail resource trade-offs? An evaluation of theory and data".Functional Ecology.32(8): 1908–1920.doi:10.1111/1365-2435.13122.ISSN0269-8463.
- ^Hill, Geoffrey E.; Johnson, James D. (November 2012)."The Vitamin A–Redox Hypothesis: A Biochemical Basis for Honest Signaling via Carotenoid Pigmentation".The American Naturalist.180(5): E127–E150.doi:10.1086/667861.ISSN0003-0147.PMID23070328.S2CID2013258.
- ^Powers, Matthew J; Hill, Geoffrey E (2021-05-03)."A Review and Assessment of the Shared-Pathway Hypothesis for the Maintenance of Signal Honesty in Red Ketocarotenoid-Based Coloration".Integrative and Comparative Biology.61(5): 1811–1826.doi:10.1093/icb/icab056.ISSN1540-7063.PMID33940618.
- ^Helfenstein, Fabrice; Losdat, Sylvain; Møller, Anders Pape; Blount, Jonathan D.; Richner, Heinz (February 2010)."Sperm of colourful males are better protected against oxidative stress".Ecology Letters.13(2): 213–222.Bibcode:2010EcolL..13..213H.doi:10.1111/j.1461-0248.2009.01419.x.ISSN1461-0248.PMID20059524.
- ^abKim, Sin-Yeon; Velando, Alberto (January 2020)."Attractive male sticklebacks carry more oxidative DNA damage in the soma and germline".Journal of Evolutionary Biology.33(1): 121–126.doi:10.1111/jeb.13552.ISSN1420-9101.PMID31610052.S2CID204702365.
- ^Liu GY, Essex A, Buchanan JT, et al. (2005)."Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity".J. Exp. Med.202(2): 209–15.doi:10.1084/jem.20050846.PMC2213009.PMID16009720.
- ^abcdefghNisar, Nazia; Li, Li; Lu, Shan; Khin, Nay Chi; Pogson, Barry J. (2015-01-05)."Carotenoid Metabolism in Plants".Molecular Plant.Plant Metabolism and Synthetic Biology.8(1): 68–82.doi:10.1016/j.molp.2014.12.007.PMID25578273.S2CID26818009.
- ^abcdefghijKUZUYAMA, Tomohisa; SETO, Haruo (2012-03-09)."Two distinct pathways for essential metabolic precursors for isoprenoid biosynthesis".Proceedings of the Japan Academy. Series B, Physical and Biological Sciences.88(3): 41–52.Bibcode:2012PJAB...88...41K.doi:10.2183/pjab.88.41.ISSN0386-2208.PMC3365244.PMID22450534.
- ^Moise, Alexander R.; Al-Babili, Salim; Wurtzel, Eleanore T. (31 October 2013)."Mechanistic aspects of carotenoid biosynthesis".Chemical Reviews.114(1): 164–93.doi:10.1021/cr400106y.PMC3898671.PMID24175570.
- ^Nisar, Nazia; Li, Li; Lu, Shan; ChiKhin, Nay; Pogson, Barry J. (5 January 2015)."Carotenoid Metabolism in Plants".Molecular Plant.8(1): 68–82.doi:10.1016/j.molp.2014.12.007.PMID25578273.S2CID26818009.
- ^Choi, Seyoung; Koo, Sangho (2005). "Efficient Syntheses of the Keto-carotenoids Canthaxanthin, Astaxanthin, and Astacene".J. Org. Chem.70(8): 3328–3331.doi:10.1021/jo050101l.PMID15823009.
External links
edit- Carotenoidsat the U.S. National Library of MedicineMedical Subject Headings(MeSH)