Cortisoneis apregnene(21-carbon)steroid hormone.It is a naturally-occurringcorticosteroidmetabolite that is also used as a pharmaceuticalprodrug.Cortisolis converted by the action of the enzymecorticosteroid 11-beta-dehydrogenase isozyme 2into the inactive metabolite cortisone, particularly in the kidneys. This is done byoxidizingthe alcohol group at carbon 11 (in the six-membered ring fused to the five-membered ring). Cortisone is converted back to the active steroid cortisol bystereospecifichydrogenationat carbon 11 by the enzyme11β-Hydroxysteroid dehydrogenase type 1,particularly in the liver.
| |
| |
| Names | |
|---|---|
| Pronunciation | /ˈkɔːrtɪsoʊn/,/ˈkɔːrtɪzoʊn/ |
| IUPAC name
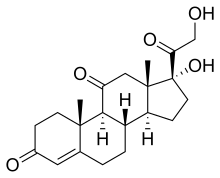
17α,21-Dihydroxypregn-4-ene-3,11,20-trione
| |
| Systematic IUPAC name
(1R,3aS,3bS,9aR,9bS,11aS)-1-Hydroxy-1-(hydroxyacetyl)-9a,11a-dimethyl-2,3,3a,3b,4,5,8,9,9a,9b,11,11a-dodecahydro-7H-cyclopenta[a]phenanthrene-7,10(1H)-dione | |
| Other names
17α,21-Dihydroxy-11-ketoprogesterone; 17α-Hydroxy-11-dehydrocorticosterone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.149 |
| KEGG | |
| MeSH | Cortisone |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C21H28O5 | |
| Molar mass | 360.450g·mol−1 |
| Melting point | 220 to 224 °C (428 to 435 °F; 493 to 497 K) |
| Pharmacology | |
| H02AB10(WHO)S01BA03(WHO) | |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
The term "cortisone" is frequently misused to mean either anycorticosteroidorhydrocortisone,which is in factcortisol.Many who speak of receiving a "cortisone shot" or taking "cortisone" are more likely receiving hydrocortisone or one of many other, much more potent synthetic corticosteroids.
Cortisone can be administered as a prodrug, meaning it has to be converted by the body (specifically the liver, converting it into cortisol) after administration to be effective. It is used to treat a variety of ailments and can be administeredintravenously,orally,intra-articularly(into a joint), ortranscutaneously.Cortisone suppresses various elements of the immune system, thus reducing inflammation and attendant pain and swelling. Risks exist, in particular in the long-term use of cortisone.[1][2]However, using cortisone only results in very mild activity, and very often more potent steroids are used instead.
Effects and uses
editCortisone itself is inactive.[3]It must be converted to cortisol by the action of11β-hydroxysteroid dehydrogenase type 1.[4]This primarily happens in the liver, the main site at which cortisone becomes cortisol after oral or systemic injection, and can thus have a pharmacological effect. After application to the skin or injection into a joint, local cells that express 11β-hydroxysteroid dehydrogenase type 1 instead convert it to active cortisol.
A cortisone injection may provide short-term pain relief and may reduce the swelling frominflammationof ajoint,tendon,orbursain, for example, the joints of theknee,elbowandshoulder[1]and into a brokencoccyx.[5]
Cortisone is used bydermatologiststo treatkeloids,[6]relieve the symptoms ofeczemaandatopic dermatitis,[7]and stop the development ofsarcoidosis.[8]
Side effects
editOral use of cortisone has a number of potential systemic adverse effects, includingasthma,hyperglycemia,insulin resistance,diabetes mellitus,osteoporosis,anxiety,depression,amenorrhoea,cataracts,glaucoma,Cushing's syndrome,increased risk of infections,andimpaired growth.[1][2]Withtopical application,it can lead to thinning of the skin, impairedwound healing,increased skin pigmentation,tendon rupture,andskin infections(includingabscesses).[9]
History
editCortisone was first identified by the American chemistsEdward Calvin Kendalland Harold L. Mason while researching at theMayo Clinic.[10][11][12]During the discovery process, cortisone was known as compound E (whilecortisolwas known as compound F).
In 1949,Philip S. Henchand colleagues discovered that large doses of injected cortisone were effective in the treatment of patients with severerheumatoid arthritis.[13]Kendall was awarded the 1950Nobel Prize for Physiology or Medicinealong withPhilip Showalter HenchandTadeusz Reichsteinfor the discovery of the structure and function ofadrenal cortexhormones including cortisone.[14][15]Both Reichstein and the team of O. Wintersteiner and J. Pfiffner had separately isolated the compound prior to the discovery made by Mason and Kendall, but failed to recognize its biological significance.[11]Mason's contributions to the crystallization and characterization of the compound have generally been forgotten outside of the Mayo Clinic.[11]
Cortisone was first produced commercially byMerck & Co.in 1948 or 1949.[13][16]On September 30, 1949,Percy Julianannounced an improvement in the process of producing cortisone frombile acids.[17]This eliminated the need to useosmium tetroxide,a rare, expensive, and dangerous chemical. In the UK in the early 1950s,John CornforthandKenneth Callowat theNational Institute for Medical Researchcollaborated withGlaxoto produce cortisone fromhecogeninfromsisalplants.[18]
Production
editCortisone is one of several end-products of a process calledsteroidogenesis.This process starts with the synthesis ofcholesterol,which then proceeds through a series of modifications in theadrenal glandto become any one of many steroid hormones. One end-product of this pathway iscortisol.For cortisol to be released from the adrenal gland, a cascade of signaling occurs.Corticotropin-releasing hormonereleased from thehypothalamusstimulates corticotrophs in theanterior pituitaryto releaseACTH,which relays the signal to the adrenal cortex. Here, thezona fasciculataandzona reticularis,in response to ACTH, secrete glucocorticoids, in particular cortisol. In various peripheral tissues, notably the kidneys, cortisol is inactivated to cortisone by theenzymecorticosteroid 11-beta-dehydrogenase isozyme 2.This is crucial because cortisol is a potentmineralocorticoidand would cause havoc with electrolyte levels (raising blood sodium and lowering blood potassium levels) and raise blood pressure if it were not inactivated in the kidneys.[4]
Because cortisone must be converted to cortisol before being active as aglucocorticoid,its activity is less than simply administering cortisol directly (80–90%).[19]
Popular culture
editAbuse and addiction to cortisone was the subject of the 1956 motion pictureBigger Than Life,produced by and starringJames Mason.Though it was a box-office flop upon its initial release,[20]many modern critics hail the film as a masterpiece and brilliant indictment of contemporary attitudes toward mental illness and addiction.[21]In 1963,Jean-Luc Godardnamed it one of the ten greatest American sound films ever made.[22]
John F. Kennedywas regularly administeredcorticosteroidssuch as cortisone as a treatment forAddison's disease.[23]
See also
editExternal links
edit- Media related toCortisoneat Wikimedia Commons
Notes
edit- ^abc"Cortisone shots".MayoClinic.com. 2010-11-16.RetrievedJuly 31,2013.
- ^ab"Prednisone and other corticosteroids: Balance the risks and benefits".MayoClinic.com. 2010-06-05.Retrieved2017-12-21.
- ^Martindale, William; Reynolds, James, eds. (1993).Martindale, The Extra Pharmacopoeia(30th ed.). Pharmaceutical Press. p. 726.ISBN978-0853693000.
- ^abCooper MS, Stewart PM (2009)."11Beta-hydroxysteroid dehydrogenase type 1 and its role in the hypothalamus-pituitary-adrenal axis, metabolic syndrome, and inflammation".J Clin Endocrinol Metab.94(12): 4645–4654.doi:10.1210/jc.2009-1412.PMID19837912.
- ^"injections and needles for coccyx pain".www.coccyx.org.
- ^Zanon, E; Jungwirth, W; Anderl, H (1992). "Cortisone jet injection as therapy of hypertrophic keloids".Handchirurgie, Mikrochirurgie, Plastische Chirurgie.24(2): 100–2.PMID1582609.
- ^"All About Atopic Dermatitis".National Eczema Association. Archived fromthe originalon 2012-01-30.Retrieved2013-05-07.
- ^Bogart, A.S.; Daniel, D.D.; Poster, K.G. (1954)."Cortisone Treatment of Sarcoidosis".Diseases of the Chest.26(2): 224–228.doi:10.1378/chest.26.2.224.PMID13182965.
- ^Cole, BJ; Schumacher (Jan–Feb 2005). "Injectable Corticosteroids in Modern Practice".Journal of the American Academy of Orthopaedic Surgeons.13(1): 37–46.CiteSeerX10.1.1.562.1931.doi:10.5435/00124635-200501000-00006.PMID15712981.S2CID18658724.
- ^"Cortisone Discovery and the Nobel Prize".Mayo Clinic.Retrieved2009-07-04.
- ^abc"I Went to See the Elephant" autobiography ofDwight J. Ingle,published by Vantage Press (1963), pg 94, 109
- ^Mason, Harold L.; Myers, Charles S.; Kendall, Edward C. (1936)."The chemistry of crystalline substances isolated from the suprarenal gland"(PDF).J. Biol. Chem.114(3): 613.doi:10.1016/S0021-9258(18)74790-X.Retrieved2014-09-07.
- ^abThomas L. Lemke; David A. Williams (2008).Foye's Principles of Medicinal Chemistry.Lippincott Williams & Wilkins. pp.889–.ISBN978-0-7817-6879-5.
- ^"The Nobel Prize in Physiology or Medicine 1950".The Nobel Prize.The Nobel Foundation. 2021.Retrieved2 April2021.
- ^Glyn, J (1998)."The discovery and early use of cortisone".J R Soc Med.91(10): 513–517.doi:10.1177/014107689809101004.PMC1296908.PMID10070369.
- ^Calvert DN (1962). "Anti-inflammatory steroids".Wis. Med. J.61:403–4.PMID13875857.
- ^Gibbons, Ray (1949). "Science gets synthetic key to rare drug; discovery is made in Chicago".Chicago Tribune.Chicago. p. 1.
- ^Quirke, Viviane (2005). "Making British Cortisone: Glaxo and the development of Corticosteroids in Britain in the 1950s–1960s".Studies in History and Philosophy of Science Part C.36(4): 645–674.doi:10.1016/j.shpsc.2005.09.001.PMID16337555.
- ^"Corticosteroid Dose Equivalents".Medscape.Retrieved20 December2016.
- ^Cossar 2011,p. 273.
- ^Halliwell 2013,pp. 159–162.
- ^Marshall, Colin (December 2, 2013)."A Young Jean-Luc Godard Picks the 10 Best American Films Ever Made (1963)".Open Culture.
- ^Altman, Lawrence (October 6, 1992)."The doctor's world; Disturbing Issue of Kennedy's Secret Illness".The New York Times.
Bibliography
edit- Bonagura J., DVM; et al. (2000).Current Veterinary Therapy.Vol. 13. pp. 321–381.
- Cossar, Harper (2011).Letterboxed: The Evolution of Widescreen Cinema.University Press of Kentucky.ISBN978-0-813-12651-7.
- Halliwell, Martin (2013).Therapeutic Revolutions: Medicine, Psychiatry, and American Culture, 1945-1970.Rutgers University Press.ISBN978-0-813-56066-3.
- Ingle DJ (October 1950)."The biologic properties of cortisone: a review".J. Clin. Endocrinol. Metab.10(10): 1312–54.doi:10.1210/jcem-10-10-1312.PMID14794756.[permanent dead link]
- Woodward R. B.; Sondheimer F.; Taub D. (1951). "The Total Synthesis of Cortisone".Journal of the American Chemical Society.73(8): 4057.Bibcode:1951JAChS..73.4057W.doi:10.1021/ja01152a551.