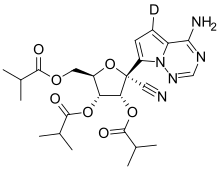
Deuremidevir,also known asVV116,is a nucleoside analogueantiviraldrug. It is administrated through oral tablets, which contain thehydrobromidesalt of this drug.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Dân đắc duy |
| Other names | VV116, JT001, mindeudesivir, renmindevir |
| Routes of administration | oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C24H30DN5O7 |
| Molar mass | 502.546g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The drug is adeuteratedtri-isobutyrateofGS-441524,theactive metaboliteofremdesivir.It was first described in a November 2020 preprint by a team including members ofWuhan Institute of Virologyand Vigonvita.[2]It completed a phase 3 trial in 2022.[3]Results from a separate Phase 3 trial conducted in mainland China from October 2022 to January 2023 suggested that deuremidevir may shorten the duration of COVID-19 symptoms in non-hospitalized adults with mild-to-moderate disease compared to placebo.[4]Junshi, which markets the drug, received conditional approval from China'sNational Medical Products Administrationin January 2023.[5][6]
In November 2023, in response to viral mutations and changing characteristics of infection, the WHO adjusted its treatment guidelines. Among other changes, the use of deuremidevir was recommended against, except for clinical trials.[7]
Research
editIn preclinical studies, a single high dose of the drug (at least 1.0 g/kg) was shown to be tolerated in rats and dogs.[8]
References
edit- ^Zhu KW (September 2023)."Deuremidevir and Simnotrelvir-Ritonavir for the Treatment of COVID-19".ACS Pharmacology & Translational Science.6(9): 1306–1309.doi:10.1021/acsptsci.3c00134.PMC10496140.PMID37705591.
- ^Yin W, Luan X, Li Z, Xie Y, Zhou Z, Liu J, et al. (November 2020). "Structural basis for repurpose and design of nucleoside drugs for treating COVID-19".bioRxiv.doi:10.1101/2020.11.01.363812.S2CID226263471.
- ^Cao Z, Gao W, Bao H, Feng H, Mei S, Chen P, et al. (February 2023)."VV116 versus Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19".The New England Journal of Medicine.388(5): 406–417.doi:10.1056/NEJMoa2208822.PMC9812289.PMID36577095.
- ^Fan X, Dai X, Ling Y, Wu L, Tang L, Peng C, et al. (February 2024). "Oral VV116 versus placebo in patients with mild-to-moderate COVID-19 in China: a multicentre, double-blind, phase 3, randomised controlled study".The Lancet Infectious Diseases.24(2): 129–139.doi:10.1016/S1473-3099(23)00577-7.PMID38006892.
- ^Devarasetti H (2023-01-30)."China's NMPA conditionally approves two oral drugs for Covid-19".Pharmaceutical Technology.Retrieved2023-02-04.
- ^Liu W, Zhang M, Hu C, Song H, Mei Y, Liu Y, et al. (November 2023)."Remdesivir Derivative VV116 Is a Potential Broad-Spectrum Inhibitor of Both Human and Animal Coronaviruses".Viruses.15(12): 2295.doi:10.3390/v15122295.PMC10748125.PMID38140536.
- ^"WHO updates its guidance on treatments for COVID-19".BMJ(Press release). 9 November 2023.
- ^Xie Y, Yin W, Zhang Y, Shang W, Wang Z, Luan X, et al. (November 2021)."Design and development of an oral remdesivir derivative VV116 against SARS-CoV-2".Cell Research.31(11): 1212–1214.doi:10.1038/s41422-021-00570-1.PMC8477624.PMID34584244.