Ferritinis a universal intracellular and extracellularproteinthat storesironand releases it in a controlled fashion. The protein is produced by almost all living organisms, includingarchaea,bacteria,algae,higher plants,andanimals.It is the primaryintracellular iron-storage proteinin bothprokaryotesandeukaryotes,keeping iron in a soluble and non-toxic form. In humans, it acts as a buffer againstiron deficiencyandiron overload.[3]
| Ferritin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
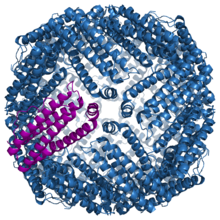 Structure of the murine ferritin complex[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Ferritin | ||||||||
| Pfam | PF00210 | ||||||||
| Pfamclan | CL0044 | ||||||||
| InterPro | IPR008331 | ||||||||
| SCOP2 | 1fha/SCOPe/SUPFAM | ||||||||
| |||||||||
| ferritin, light polypeptide | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | FTL | ||||||
| NCBI gene | 2512 | ||||||
| HGNC | 3999 | ||||||
| OMIM | 134790 | ||||||
| RefSeq | NM_000146 | ||||||
| UniProt | P02792 | ||||||
| Other data | |||||||
| Locus | Chr. 19q13.3–13.4 | ||||||
| |||||||
| ferritin, heavy polypeptide 1 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | FTH1 | ||||||
| Alt. symbols | FTHL6 | ||||||
| NCBI gene | 2495 | ||||||
| HGNC | 3976 | ||||||
| OMIM | 134770 | ||||||
| RefSeq | NM_002032 | ||||||
| UniProt | P02794 | ||||||
| Other data | |||||||
| Locus | Chr. 11q13 | ||||||
| |||||||
| ferritin mitochondrial | |||||||
|---|---|---|---|---|---|---|---|
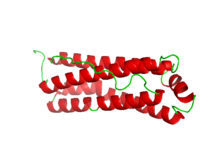 Crystallographic structure of mitochondrial ferritin.[2] | |||||||
| Identifiers | |||||||
| Symbol | FTMT | ||||||
| NCBI gene | 94033 | ||||||
| HGNC | 17345 | ||||||
| OMIM | 608847 | ||||||
| RefSeq | NM_177478 | ||||||
| UniProt | Q8N4E7 | ||||||
| Other data | |||||||
| Locus | Chr. 5q23.1 | ||||||
| |||||||
Ferritin is found in most tissues as acytosolicprotein, but small amounts are secreted into theserumwhere it functions as an iron carrier. Plasma ferritin is also an indirectmarkerof the total amount of iron stored in the body; hence, serum ferritin is used as adiagnostic testforiron-deficiency anemiaandiron overload.[4]Aggregated ferritin transforms into a water insoluble, crystalline and amorphous form of storage iron calledhemosiderin.[5]
Ferritin is aglobular proteincomplex consisting of 24protein subunitsforming a hollow spherical nanocage with multiple metal–protein interactions.[6]Ferritin with iron removed is calledapoferritin.[7]: e10
Gene
editFerritin genes are highlyconservedbetween species. All vertebrate ferritin genes have threeintronsand fourexons.[8]In human ferritin, introns are present betweenamino acidresidues14 and 15, 34 and 35, and 82 and 83; in addition, there are one to two hundreduntranslated basesat either end of the combined exons.[9]Thetyrosineresidue at amino acid position 27 is thought to be associated withbiomineralization.[10]
Protein structure
editFerritin is a hollowglobular proteinof mass 474kDaand comprising 24 subunits. Typically it has internal and external diameters of about 8 and 12 nm, respectively.[11]The nature of these subunits varies by class of organism:
- Invertebrates,the subunits are of two types,light (L)andheavy (H),which have apparent molecular mass of 19 kDa and 21 kDa, respectively; their sequences arehomologous(about 50% identical).[9]
- Amphibianshave an additional ( "M" ) type of ferritin.[12]
- Plantsandbacteriahave a single ferritin; it most closely resembles the vertebrate H-type.[12]
- In thegastropodsof thegenusLymnaea,two types have been recovered, fromsomaticcells and theyolk,respectively (see below).[12]
- In the pearl oysterPinctada fucata,an additional subunit resemblingLymnaeasoma ferritin is associated with shell formation.[13]
- In the parasiteSchistosoma,two types are present: one in males, the other in females.[12]
All the aforementioned ferritins are similar, in terms of their primary sequence, with the vertebrate H-type.[12]InE. coli,a 20% similarity to human H-ferritin is observed.[12]Some ferritin complexes in vertebrates arehetero-oligomersof two highly relatedgeneproducts with slightly differentphysiologicalproperties. The ratio of the twohomologousproteins in the complex depends on the relative expression levels of the two genes.
Cytosolic ferritinshell (apoferritin) is a heteropolymer of 24 subunits of heavy (H) and light (L) peptides that form a hollow spherical nanocage that covers an iron core composed ofcrystallitestogether withphosphateandhydroxideions. The resulting particle is similar toferrihydrite(5Fe2O3·9H2O). Each ferritin complex can store about 4500 iron (Fe3+) ions.[9][12]The proportion of H to L subunits varies in ferritin from different tissues, explaining its heterogeneity on isoelectric focusing. L-rich ferritins (from spleen and liver) are more basic than H-rich ferritins (from heart and red blood cells).
Serum ferritin,which is typically iron-poor, consists almost exclusively of L subunits. Serum ferritin is heterogeneous due to glycosylation. The glycosylation and direct relationship of serum ferritin concentration to iron storage in macrophages suggest it is secreted by macrophages in response to changing iron levels.
Humanmitochondrial ferritin,MtF, was found to express as apro-protein.[14]When a mitochondrion takes it up, it processes it into a mature protein similar to the ferritins found in thecytoplasm,which it assembles to form functional ferritin shells. Unlike other human ferritins, it is a homopolymer of H type ferritin and appears to have no introns (intronless) in its genetic code. The mitochondrial ferritin'sRamachandran plot[15]shows its structure to be mainlyalpha helicalwith a low prevalence ofbeta sheets.It accumulates in large amounts in the erythroblasts of subjects with impaired heme synthesis.
Function
editIron storage
editFerritin is present in every cell type.[9]It serves to store iron in a non-toxic form, to deposit it in a safe form, and to transport it to areas where it is required.[16]The function and structure of the expressed ferritin protein varies in different cell types. This is controlled primarily by the amount and stability ofmessenger RNA(mRNA), but also by changes in how the mRNA is stored and how efficiently it is transcribed.[9]One major trigger for the production of many ferritins is the mere presence of iron;[9]an exception is the yolk ferritin ofLymnaea sp.,which lacks an iron-responsive unit.[12]
Free iron istoxictocellsas it acts as acatalystin the formation offree radicalsfromreactive oxygen speciesvia theFenton reaction.[17]Hence vertebrates have an elaborate set of protective mechanisms to bind iron in varioustissuecompartments[discuss].Within cells, iron is stored in a protein complex as ferritin or the related complexhemosiderin.Apoferritin binds to free ferrous iron and stores it in the ferric state. As ferritin accumulates within cells of thereticuloendothelial system,protein aggregates are formed as hemosiderin. Iron in ferritin or hemosiderin can be extracted for release by the RE cells, although hemosiderin is less readily available. Understeady-stateconditions, the level of ferritin in theblood serumcorrelates with total body stores of iron; thus, the serum ferritin FR5Rl is the most convenient laboratory test to estimate iron stores.[citation needed]
Because iron is an important mineral in mineralization, ferritin is employed in the shells of organisms such asmolluscsto control the concentration and distribution of iron, thus sculpting shell morphology and colouration.[18][19]It also plays a role in thehaemolymphof thepolyplacophora,where it serves to rapidly transport iron to the mineralizingradula.[20]
Iron is released from ferritin for use by ferritin degradation, which is performed mainly bylysosomes.[21]
Ferroxidase activity
editVertebrate ferritin consists of two or three subunits which are named based on their molecular weight: L "light", H "heavy", and M "middle" subunits. The M subunit has only been reported in bullfrogs. In bacteria and archaea, ferritin consists of one subunit type.[22]H and M subunits of eukaryotic ferritin and all subunits of bacterial and archaeal ferritin are H-type and have ferroxidase activity, which means they are able to convert iron from the ferrous (Fe2+) to ferric (Fe3+) forms. This limits the deleterious reaction which occurs between ferrous iron andhydrogen peroxideknown as the Fenton reaction which produces the highly damaginghydroxyl radical.The ferroxidase activity occurs at a diiron binding site in the middle of each H-type subunits.[22][23]After oxidation of Fe(II), the Fe(III) product stays metastably in the ferroxidase center and is displaced by Fe(II),[23][24]a mechanism that appears to be common among ferritins of all three domains of life.[22]The light chain of ferritin has no ferroxidase activity but may be responsible for the electron transfer across the protein cage.[25]
Immune response
editFerritin concentrations increase drastically in the presence of an infection or cancer.Endotoxinsare an up-regulator of the gene coding for ferritin, thus causing the concentration of ferritin to rise. By contrast, organisms such asPseudomonas,although possessing endotoxin, cause plasma ferritin levels to drop significantly within the first 48 hours of infection. Thus, the iron stores of the infected body are denied to the infective agent, impeding its metabolism.[26]
Stress response
editThe concentration of ferritin has been shown to increase in response to stresses such asanoxia,[27]which implies that it is anacute phase protein.[28]
Mitochondria
editMitochondrial ferritinhas many roles pertaining to molecular function. It participates in ferroxidase activity, binding, iron ion binding, oxidoreductase activity, ferric iron binding, metal ion binding as well as transition metal binding. Within the realm of biological processes it participates in oxidation-reduction, iron ion transport across membranes and cellular iron ion homeostasis.[citation needed]
Yolk
editIn some snails, the protein component of the egg yolk is primarily ferritin.[29]This is a different ferritin, with a different genetic sequence, from the somatic ferritin. It is produced in the midgut glands and secreted into the haemolymph, whence it is transported to the eggs.[29]
Tissue distribution
editIn vertebrates, ferritin is usually found within cells, although it is also present in smaller quantities in the plasma.[26]
Diagnostic uses
editSerumferritin levels are measured inmedical laboratoriesas part of the iron studies workup foriron-deficiency anemia.[6]They are measured in nanograms per milliliter (ng/mL) or micrograms per liter (μg/L); the two units are equivalent.
The ferritin levels measured usually have a direct correlation with the total amount of iron stored in the body. However, ferritin levels may be artificially high in cases ofanemia of chronic disease,where ferritin is elevated in its capacity as an inflammatoryacute phase proteinand not as a marker for iron overload.[citation needed]
Normal ranges
editA normal ferritin blood level, referred to as thereference intervalis determined by manytesting laboratories.The ranges for ferritin can vary between laboratories but typical ranges would be between 40 and 300 ng/mL (=μg/L) for males, and 20–200 ng/mL (=μg/L) for females.[30]
| Adult males | 40–300 ng/mL (μg/L)[30] |
| Adult females | 20–200 ng/mL (μg/L)[30] |
| Children(6 months to 15 years) | 50–140 ng/mL (μg/L) |
| Infants(1 to 5 months) | 50–200 ng/mL (μg/L) |
| Neonates | 25–200 ng/mL (μg/L) |
Deficiency
editAccording to a 2014 review in theNew England Journal of Medicinestated that a ferritin level below 30 ng/mL indicatesiron deficiency,while a level below 10 ng/mL indicates iron-deficiency anemia.[30]A 2020 World Health Organization guideline states that ferritin indicates iron deficiency below 12 ng/mL in apparently-healthy children under 5 and 15 ng/mL in apparently-healthy individuals of 5 and over.[31]
Some studies suggest that women withfatigueand ferritin below 50 ng/mL see reduced fatigue after iron supplementation.[32][33]
In the setting of anemia, low serum ferritin is the most specific lab finding foriron-deficiency anemia.[34]However it is less sensitive, since its levels are increased in the blood by infection or any type of chronic inflammation,[35]and these conditions may convert what would otherwise be a low level of ferritin from lack of iron, into a value in the normal range. For this reason, low ferritin levels carry more information than those in the normal range. Afalsely lowblood ferritin (equivalent to afalse positivetest) is very uncommon,[35]but can result from ahook effectof the measuring tools in extreme cases.[36]
Low ferritin may also indicatehypothyroidism,[37]vitamin C deficiencyorceliac disease.[citation needed]
Low serum ferritin levels are seen in some patients withrestless legs syndrome,not necessarily related to anemia, but perhaps due to low iron stores short of anemia.[38][39]
Vegetarianismis not a cause of low serum ferritin levels, according to the American Dietetic Association's position in 2009: "Incidence of iron-deficiency anemia among vegetarians is similar to that of non-vegetarians. Although vegetarian adults have lower iron stores than non-vegetarians, their serum ferritin levels are usually within the normal range."[40]
Excess
editIf ferritin is high, there is iron in excess or else there is an acute inflammatory reaction in which ferritin is mobilized without iron excess. For example, ferritins may be high in infection without signaling body iron overload.
Ferritin is also used as amarkerforiron overload disorders,such ashemochromatosisorhemosiderosis.Adult-onset Still's disease,someporphyrias,andhemophagocytic lymphohistiocytosis/macrophage activation syndromeare diseases in which the ferritin level may be abnormally raised.
As ferritin is also anacute-phase reactant,it is often elevated in the course of disease. A normalC-reactive proteincan be used to exclude elevated ferritin caused by acute phase reactions.[citation needed]
Ferritin has been shown to be elevated in some cases ofCOVID-19and may correlate with worse clinical outcome.[41][42]Ferritin andIL-6are considered to be possible immunological biomarkers for severe and fatal cases of COVID-19. Ferritin and C-reactive protein may be possible screening tools for early diagnosis ofsystemic inflammatory response syndromein cases of COVID-19.[43][44]
According to a study ofanorexia nervosapatients, ferritin can be elevated during periods of acutemalnourishment,perhaps due to iron going into storage as intravascular volume and thus the number of red blood cells falls.[45]
Another study suggests that due to the catabolic nature of anorexia nervosa, isoferritins may be released. Furthermore, ferritin has significant non-storage roles within the body, such as protection fromoxidative damage.The rise of these isoferritins may contribute to an overall increase in ferritin concentration. The measurement of ferritin throughimmunoassayor immunoturbidimeteric methods may also be picking up these isoferritins thus not a true reflection of iron storage status.[46]
Studies reveal that a transferrin saturation (serum iron concentration ÷ total iron binding capacity) over 60 percent in men and over 50 percent in women identified the presence of an abnormality in iron metabolism (hereditary hemochromatosis,heterozygotes, and homozygotes) with approximately 95 percent accuracy. This finding helps in the early diagnosis of hereditary hemochromatosis, especially while serum ferritin still remains low. The retained iron in hereditary hemochromatosis is primarily deposited in parenchymal cells, with reticuloendothelial cell accumulation occurring very late in the disease. This is in contrast to transfusional iron overload in which iron deposition occurs first in the reticuloendothelial cells and then in parenchymal cells. This explains why ferritin levels remain relative low in hereditary hemochromatosis, while transferrin saturation is high.[47][48]
In chronic liver diseases
editHematological abnormalities often associate with chronic liver diseases. Both iron overload and iron deficient anemia have been reported in patients with liver cirrhosis.[49][50]The former is mainly due to reducedhepcidinlevel caused by the decreased synthetic capacity of the liver, while the latter is due to acute and chronic bleeding caused byportal hypertension.Inflammation is also present in patients with advanced chronic liver disease. As a consequence, elevated hepatic and serum ferritin levels are consistently reported in chronic liver diseases.[51][52][53]
Studies showed association between high serum ferritin levels and increased risk of short-term mortality in cirrhotic patients with acute decompensation[54]and acute-on-chronic liver failure.[55]An other study found association between high serum ferritin levels and increased risk of long-term mortality in compensated and stable decompensated cirrhotic patients.[56]The same study demonstrated that increased serum ferritin levels could predict the development of bacterial infection in stable decompensated cirrhotic patients, while in compensated cirrhotic patients the appearance of the very first acute decompensation episode showed higher incidence in patients with low serum ferritin levels. This latter finding was explained by the association between chronic bleeding and increased portal pressure.[56]
Discovery
editFerritin was discovered in 1937 by theCzechoslovakianscientistVilém Laufberger.[57][7]: e9 Sam GranickandLeonor Michaelisproduced apoferritin in 1942[7]: e10
Applications
editFerritin is used inmaterials scienceas a precursor in making ironnanoparticles(NP) forcarbon nanotubegrowth bychemical vapor deposition.
Cavities formed by ferritin and mini-ferritins (Dps) proteins have been successfully used as the reaction chamber for the fabrication of metal nanoparticles.[58][59][60][61]Proteinshells served as a template to restrain particle growth and as a coating to prevent coagulation/aggregation between NPs. Using various sizes of protein shells, various sizes of NPs can be easily synthesized for chemical, physical and bio-medical applications.[6][62]
Experimental COVID-19 vaccines have been produced that display thespike protein's receptor binding domain on the surface of ferritin nanoparticles.[63]
Notes
editThe primary peptide sequence of human ferritin is:[64]
MTTASTSQVR QNYHQDSEAA INRQINLELY ASYVYLSMSY YFDRDDVALK NFAKYFLHQS HEEREHAEKL MKLQNQRGGR IFLQDIKKPD CDDWESGLNA MECALHLEKN VNQSLLEFPS PISPSPSCWH HYTTNRPQPQ HHLLRPRRRK RPHSIPTPIL IFRSP.
See also
editReferences
edit- ^PDB:1lb3;Granier T, Langlois d'Estaintot B, Gallois B, Chevalier JM, Précigoux G, Santambrogio P, et al. (January 2003). "Structural description of the active sites of mouse L-chain ferritin at 1.2 A resolution".Journal of Biological Inorganic Chemistry.8(1–2): 105–111.doi:10.1007/s00775-002-0389-4.PMID12459904.S2CID20756710.
- ^PDB:1r03;Langlois d'Estaintot B, Santambrogio P, Granier T, Gallois B, Chevalier JM, Précigoux G, et al. (July 2004). "Crystal structure and biochemical properties of the human mitochondrial ferritin and its mutant Ser144Ala".Journal of Molecular Biology.340(2): 277–293.doi:10.1016/j.jmb.2004.04.036.PMID15201052.
- ^Casiday R, Frey R."Iron Use and Storage in the Body: Ferritin and Molecular Representations".Department of Chemistry,Washington University in St. Louis.
- ^Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV (August 2010)."Serum ferritin: Past, present and future".Biochimica et Biophysica Acta (BBA) - General Subjects.1800(8): 760–769.doi:10.1016/j.bbagen.2010.03.011.PMC2893236.PMID20304033.
- ^MacKenzie EL, Iwasaki K, Tsuji Y (June 2008)."Intracellular iron transport and storage: from molecular mechanisms to health implications".Antioxidants & Redox Signaling.10(6): 997–1030.doi:10.1089/ars.2007.1893.PMC2932529.PMID18327971.
- ^abcTheil EC (2012)."Ferritin protein nanocages-the story".Nanotechnology Perceptions.8(1): 7–16.doi:10.4024/N03TH12A.ntp.08.01.PMC3816979.PMID24198751.
- ^abcKresge N, Simoni RD, Hill RL (2004)."The Characterization of Ferritin and Apoferritin by Leonor Michaelis and Sam Granick".Journal of Biological Chemistry.279(49). Elsevier BV: e9–e11.doi:10.1016/s0021-9258(20)69471-6.ISSN0021-9258.
- ^Torti FM, Torti SV (May 2002)."Regulation of ferritin genes and protein".Blood.99(10): 3505–3516.doi:10.1182/blood.V99.10.3505.PMID11986201.
- ^abcdefTheil EC (1987). "Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms".Annual Review of Biochemistry.56(1): 289–315.doi:10.1146/annurev.bi.56.070187.001445.PMID3304136.
- ^De Zoysa M, Lee J (September 2007). "Two ferritin subunits from disk abalone (Haliotis discus discus): cloning, characterization and expression analysis".Fish & Shellfish Immunology.23(3): 624–635.Bibcode:2007FSI....23..624D.doi:10.1016/j.fsi.2007.01.013.PMID17442591.
- ^"Ferritin Structure and Its Biomedical Implications".Metallic BioNano Particles.Universidad de Granada. Archived fromthe originalon 2016-08-27.Retrieved2016-01-16.
- ^abcdefghAndrews SC, Arosio P, Bottke W, Briat JF, von Darl M, Harrison PM, et al. (1992). "Structure, function, and evolution of ferritins".Journal of Inorganic Biochemistry.47(3–4): 161–174.doi:10.1016/0162-0134(92)84062-R.PMID1431878.
- ^Zhang Y, Meng Q, Jiang T, Wang H, Xie L, Zhang R (May 2003). "A novel ferritin subunit involved in shell formation from the pearl oyster (Pinctada fucata)".Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology.135(1): 43–54.doi:10.1016/S1096-4959(03)00050-2.PMID12781972.
- ^Levi S, Corsi B, Bosisio M, Invernizzi R, Volz A, Sanford D, et al. (July 2001)."A human mitochondrial ferritin encoded by an intronless gene".The Journal of Biological Chemistry.276(27): 24437–24440.doi:10.1074/jbc.C100141200.PMID11323407.
- ^Lovell SC, Davis IW, Arendall WB, de Bakker PI, Word JM, Prisant MG, et al. (February 2003)."Structure validation by Calpha geometry: phi,psi and Cbeta deviation"(PDF).Proteins.50(3): 437–450.doi:10.1002/prot.10286.PMID12557186.S2CID8358424.Archived fromthe original(PDF)on 12 October 2012.
MolProbity Ramachandran analysis
- ^Seckback J (1982). "Ferreting out the secrets of plant ferritin - A review".Journal of Plant Nutrition.5(4–7): 369–394.Bibcode:1982JPlaN...5..369S.doi:10.1080/01904168209362966.
- ^Orino K, Lehman L, Tsuji Y, Ayaki H, Torti SV, Torti FM (July 2001)."Ferritin and the response to oxidative stress".The Biochemical Journal.357(Pt 1): 241–247.doi:10.1042/0264-6021:3570241.PMC1221947.PMID11415455.
- ^Jackson DJ, Wörheide G, Degnan BM (September 2007)."Dynamic expression of ancient and novel molluscan shell genes during ecological transitions".BMC Evolutionary Biology.7(1): 160.Bibcode:2007BMCEE...7..160J.doi:10.1186/1471-2148-7-160.PMC2034539.PMID17845714.
- ^Yano M, Nagai K, Morimoto K, Miyamoto H (June 2006). "Shematrin: a family of glycine-rich structural proteins in the shell of the pearl oyster Pinctada fucata".Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology.144(2): 254–262.doi:10.1016/j.cbpb.2006.03.004.PMID16626988.
- ^Kyung-Suk K, Webb J, Macey D (1986). "Properties and role of ferritin in the hemolymph of the chiton Clavarizona hirtosa".Biochimica et Biophysica Acta (BBA) - General Subjects.884(3): 387–394.doi:10.1016/0304-4165(86)90188-1.
- ^Zhang Y, Mikhael M, Xu D, Li Y, Soe-Lin S, Ning B, et al. (October 2010). "Lysosomal proteolysis is the primary degradation pathway for cytosolic ferritin and cytosolic ferritin degradation is necessary for iron exit".Antioxidants & Redox Signaling.13(7): 999–1009.doi:10.1089/ars.2010.3129.PMID20406137.
- ^abcHonarmand Ebrahimi K, Hagedoorn PL, Hagen WR (January 2015)."Unity in the biochemistry of the iron-storage proteins ferritin and bacterioferritin".Chemical Reviews.115(1): 295–326.doi:10.1021/cr5004908.PMID25418839.
- ^abHonarmand Ebrahimi K, Bill E, Hagedoorn PL, Hagen WR (November 2012). "The catalytic center of ferritin regulates iron storage via Fe(II)-Fe(III) displacement".Nature Chemical Biology.8(11): 941–948.doi:10.1038/nchembio.1071.PMID23001032.
- ^Watt RK (March 2013). "A unified model for ferritin iron loading by the catalytic center: implications for controlling" free iron "during oxidative stress".ChemBioChem.14(4): 415–419.doi:10.1002/cbic.201200783.PMID23404831.S2CID41485685.
- ^Carmona U, Li L, Zhang L, Knez M (December 2014). "Ferritin light-chain subunits: key elements for the electron transfer across the protein cage".Chemical Communications.50(97): 15358–15361.doi:10.1039/c4cc07996e.PMID25348725.
- ^abOng DS, Wang L, Zhu Y, Ho B, Ding JL (2005). "The response of ferritin to LPS and acute phase of Pseudomonas infection".Journal of Endotoxin Research.11(5): 267–280.doi:10.1179/096805105X58698.PMID16262999.
- ^Larade K, Storey KB (March 2004)."Accumulation and translation of ferritin heavy chain transcripts following anoxia exposure in a marine invertebrate".The Journal of Experimental Biology.207(Pt 8): 1353–1360.doi:10.1242/jeb.00872.PMID15010486.
- ^Beck G, Ellis TW, Habicht GS, Schluter SF, Marchalonis JJ (January 2002). "Evolution of the acute phase response: iron release by echinoderm (Asterias forbesi) coelomocytes, and cloning of an echinoderm ferritin molecule".Developmental and Comparative Immunology.26(1): 11–26.doi:10.1016/S0145-305X(01)00051-9.PMID11687259.
- ^abBottke W, Burschyk M, Volmer J (December 1988). "On the origin of the yolk protein ferritin in snails".Roux's Archives of Developmental Biology.197(7): 377–382.doi:10.1007/BF00398988.PMID28305744.S2CID34033340.
- ^abcdCamaschella C (May 2015). Longo DL (ed.). "Iron-deficiency anemia".The New England Journal of Medicine.372(19): 1832–1843.doi:10.1056/NEJMra1401038.PMID25946282.S2CID17628280.
- ^WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations.Geneva, Switzerland: World Health Organization. 2020.ISBN978-92-4-000012-4.OCLC1265083396.
- ^Verdon F, Burnand B, Stubi CL, Bonard C, Graff M, Michaud A, et al. (May 2003)."Iron supplementation for unexplained fatigue in non-anaemic women: double blind randomised placebo controlled trial".BMJ.326(7399): 1124–0.doi:10.1136/bmj.326.7399.1124.PMC156009.PMID12763985.
- ^Vaucher P, Druais PL, Waldvogel S, Favrat B (August 2012)."Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial".CMAJ.184(11): 1247–1254.doi:10.1503/cmaj.110950.PMC3414597.PMID22777991.
- ^Guyatt GH, Patterson C, Ali M, Singer J, Levine M, Turpie I, et al. (March 1990). "Diagnosis of iron-deficiency anemia in the elderly".The American Journal of Medicine.88(3): 205–209.doi:10.1016/0002-9343(90)90143-2.PMID2178409.
- ^abFirkin F, Rush B (1997)."Interpretation of biochemical tests for iron deficiency: diagnostic difficulties related to limitations of individual tests".Australian Prescriber.20:74–6.doi:10.18773/austprescr.1997.063.Archived fromthe originalon 2012-03-25.
- ^Burnett D, Crocker JR (1999).The Science of Laboratory Diagnosis.ISIS Medical Media. p. 341.ISBN978-1-899066-62-9.
- ^R V, Dhiman P, Kollipaka R, P S, V H (August 2022)."Association of Hypothyroidism With Low Serum Ferritin Levels and Iron-Deficiency Anemia During the First Trimester of Pregnancy".Cureus.14(8): e28307.doi:10.7759/cureus.28307.PMC9498961.PMID36158423.
- ^Kryger MH, Otake K, Foerster J (March 2002). "Low body stores of iron and restless legs syndrome: a correctable cause of insomnia in adolescents and teenagers".Sleep Medicine.3(2): 127–132.doi:10.1016/S1389-9457(01)00160-5.PMID14592231.
- ^Mizuno S, Mihara T, Miyaoka T, Inagaki T, Horiguchi J (March 2005)."CSF iron, ferritin and transferrin levels in restless legs syndrome".Journal of Sleep Research.14(1): 43–47.doi:10.1111/j.1365-2869.2004.00403.x.PMID15743333.S2CID12959227.
- ^Craig WJ, Mangels AR (July 2009). "Position of the American Dietetic Association: vegetarian diets".Journal of the American Dietetic Association.109(7): 1266–1282.doi:10.1016/j.jada.2009.05.027.PMID19562864.S2CID7906168.
- ^Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. (May 2020)."Clinical and immunological features of severe and moderate coronavirus disease 2019".The Journal of Clinical Investigation.130(5): 2620–2629.doi:10.1172/JCI137244.PMC7190990.PMID32217835.
- ^Zachariah M, Maamoun H, Milano L, Rayman MP, Meira LB, Agouni A (June 2021)."Endoplasmic reticulum stress and oxidative stress drive endothelial dysfunction induced by high selenium".Journal of Cellular Physiology.236(6): 4348–4359.doi:10.1080/20905068.2020.1870788.PMC8108185.PMID33241572.
- ^Melo AK, Milby KM, Caparroz AL, Pinto AC, Santos RR, Rocha AP, et al. (29 June 2021)."Biomarkers of cytokine storm as red flags for severe and fatal COVID-19 cases: A living systematic review and meta-analysis".PLOS ONE.16(6): e0253894.Bibcode:2021PLoSO..1653894M.doi:10.1371/journal.pone.0253894.PMC8241122.PMID34185801.
- ^Dance A (10 April 2020)."What is a cytokine storm?".Knowable Magazine.Annual Reviews.Retrieved9 August2021.
- ^Kennedy A, Kohn M, Lammi A, Clarke S (August 2004). "Iron status and haematological changes in adolescent female inpatients with anorexia nervosa".Journal of Paediatrics and Child Health.40(8): 430–432.doi:10.1111/j.1440-1754.2004.00432.x.PMID15265182.S2CID26269832.
- ^Tran J, Story C, Moore D, Metz M (September 2013)."Unexpected increased ferritin concentration in patients with anorexia nervosa".Annals of Clinical Biochemistry.50(Pt 5): 504–506.doi:10.1177/0004563213490289.PMID23897102.S2CID9927714.
- ^Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS (July 2011)."Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases".Hepatology.54(1): 328–343.doi:10.1002/hep.24330.PMC3149125.PMID21452290.
- ^"Hemochromatosis".guidelinecentral.com.
- ^Cotler SJ, Bronner MP, Press RD, Carlson TH, Perkins JD, Emond MJ, et al. (August 1998). "End-stage liver disease without hemochromatosis associated with elevated hepatic iron index".Journal of Hepatology.29(2): 257–262.doi:10.1016/S0168-8278(98)80011-1.PMID9722207.
- ^Gkamprela E, Deutsch M, Pectasides D (2017)."Iron deficiency anemia in chronic liver disease: etiopathogenesis, diagnosis and treatment".Annals of Gastroenterology.30(4): 405–413.doi:10.20524/aog.2017.0152.PMC5479992.PMID28655976.
- ^Guyader D, Thirouard AS, Erdtmann L, Rakba N, Jacquelinet S, Danielou H, et al. (April 2007). "Liver iron is a surrogate marker of severe fibrosis in chronic hepatitis C".Journal of Hepatology.46(4): 587–595.doi:10.1016/j.jhep.2006.09.021.PMID17156889.
- ^Adams PC (1998). "Iron overload in viral and alcoholic liver disease".Journal of Hepatology.28(Suppl 1): 19–20.doi:10.1016/S0168-8278(98)80370-X.PMID9575444.
- ^Eng SC, Taylor SL, Reyes V, Raaka S, Berger J, Kowdley KV (June 2005). "Hepatic iron overload in alcoholic end-stage liver disease is associated with iron deposition in other organs in the absence of HFE-1 hemochromatosis".Liver International.25(3): 513–517.doi:10.1111/j.1478-3231.2005.01004.x.PMID15910487.S2CID23125116.
- ^Maiwall R, Kumar S, Chaudhary AK, Maras J, Wani Z, Kumar C, et al. (July 2014). "Serum ferritin predicts early mortality in patients with decompensated cirrhosis".Journal of Hepatology.61(1): 43–50.doi:10.1016/j.jhep.2014.03.027.PMID24681346.
- ^Maras JS, Maiwall R, Harsha HC, Das S, Hussain MS, Kumar C, et al. (April 2015)."Dysregulated iron homeostasis is strongly associated with multiorgan failure and early mortality in acute-on-chronic liver failure".Hepatology.61(4): 1306–1320.doi:10.1002/hep.27636.PMID25475192.
- ^abTornai D, Antal-Szalmas P, Tornai T, Papp M, Tornai I, Sipeki N, et al. (March 2021)."Abnormal ferritin levels predict development of poor outcomes in cirrhotic outpatients: a cohort study".BMC Gastroenterology.21(1): 94.doi:10.1186/s12876-021-01669-w.PMC7923668.PMID33653274.
- ^Laufberger V (1937). "Sur la Cristallisation de la Ferritine".Bulletin de la Société de Chimie Biologique.19:1575–1582.ISSN0037-9042.
- ^Kasyutich O, Ilari A, Fiorillo A, Tatchev D, Hoell A, Ceci P (March 2010). "Silver ion incorporation and nanoparticle formation inside the cavity of Pyrococcus furiosus ferritin: structural and size-distribution analyses".Journal of the American Chemical Society.132(10): 3621–3627.doi:10.1021/ja910918b.PMID20170158.
- ^Uchida M, Flenniken ML, Allen M, Willits DA, Crowley BE, Brumfield S, et al. (December 2006). "Targeting of cancer cells with ferrimagnetic ferritin cage nanoparticles".Journal of the American Chemical Society.128(51): 16626–16633.doi:10.1021/ja0655690.PMID17177411.
- ^Li M, Viravaidya C, Mann S (September 2007). "Polymer-mediated synthesis of ferritin-encapsulated inorganic nanoparticles".Small.3(9): 1477–1481.doi:10.1002/smll.200700199.PMID17768776.
- ^Ueno T, Suzuki M, Goto T, Matsumoto T, Nagayama K, Watanabe Y (May 2004). "Size-selective olefin hydrogenation by a Pd nanocluster provided in an apo-ferritin cage".Angewandte Chemie.43(19): 2527–2530.doi:10.1002/anie.200353436.PMID15127443.
- ^Stanford's Single-Dose Nanoparticle Vaccine for COVID-19.On: SciTechDaily. January 10, 2021. Source: Stanford University
- ^Wang W, Huang B, Zhu Y, Tan W, Zhu M (March 2021)."Ferritin nanoparticle-based SARS-CoV-2 RBD vaccine induces a persistent antibody response and long-term memory in mice".Cellular & Molecular Immunology.18(3): 749–751.doi:10.1038/s41423-021-00643-6.PMC7880661.PMID33580169.
- ^"Ferritin - Homo sapiens (Human)".UniProt.Q8TD27.
External links
edit- Ferritinsat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
- Ferritinat Lab Tests Online
- Overview of all the structural information available in thePDBforUniProt:P02792(Ferritin light chain) at thePDBe-KB.
- Overview of all the structural information available in thePDBforUniProt:P02794(Ferritin heavy chain) at thePDBe-KB.
- Overview of all the structural information available in thePDBforUniProt:Q8N4E7(Ferritin, mitochondrial) at thePDBe-KB.