Glycolipidsarelipidswith acarbohydrateattached by aglycosidic (covalent) bond.[1]Their role is to maintain the stability of thecell membraneand to facilitatecellularrecognition, which is crucial to theimmune responseand in the connections that allow cells to connect to one another to formtissues.[2]Glycolipids are found on the surface of alleukaryoticcell membranes, where they extend from the phospholipid bilayer into the extracellular environment.[2]
Structure
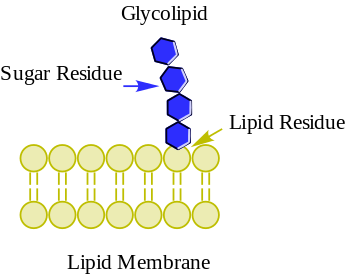
editThe essential feature of a glycolipid is the presence of amonosaccharideoroligosaccharidebound to a lipidmoiety.The most common lipids in cellular membranes areglycerolipidsandsphingolipids,which haveglycerolor asphingosinebackbones, respectively.Fatty acidsare connected to this backbone, so that the lipid as a whole has a polar head and a non-polar tail. The lipid bilayer of thecell membraneconsists of two layers of lipids, with the inner and outer surfaces of the membrane made up of the polar head groups, and the inner part of the membrane made up of the non-polar fatty acid tails.
The saccharides that are attached to the polar head groups on the outside of the cell are theligandcomponents of glycolipids, and are likewise polar, allowing them to be soluble in the aqueous environment surrounding the cell.[3]The lipid and the saccharide form aglycoconjugatethrough aglycosidic bond,which is acovalent bond.The anomeric carbon of the sugar binds to a freehydroxyl groupon the lipid backbone. The structure of these saccharides varies depending on the structure of the molecules to which they bind.
Metabolism
editGlycosyltransferases
editEnzymes calledglycosyltransferaseslink the saccharide to the lipid molecule, and also play a role in assembling the correctoligosaccharideso that the right receptor can be activated on the cell which responds to the presence of the glycolipid on the surface of the cell. The glycolipid is assembled in theGolgi apparatusand embedded in the surface of avesiclewhich is then transported to the cell membrane. The vesicle merges with the cell membrane so that the glycolipid can be presented on the cell's outside surface.[4]
Glycoside hydrolases
editGlycoside hydrolasescatalyze the breakage of glycosidic bonds. They are used to modify the oligosaccharide structure of theglycanafter it has been added onto the lipid. They can also remove glycans from glycolipids to turn them back into unmodified lipids.[5]
Defects in metabolism
editSphingolipidosesare a group of diseases that are associated with the accumulation of sphingolipids which have not been degraded correctly, normally due to a defect in a glycoside hydrolase enzyme. Sphingolipidoses are typically inherited, and their effects depend on which enzyme is affected, and the degree of impairment. One notable example isNiemann–Pick diseasewhich can cause pain and damage to neural networks.[6]
Function
editCell–cell interactions
editThe main function of glycolipids in the body is to serve as recognition sites for cell–cell interactions. The saccharide of the glycolipid will bind to a specific complementary carbohydrate or to alectin(carbohydrate-binding protein), of a neighboring cell. The interaction of these cell surface markers is the basis of cell recognitions, and initiates cellular responses that contribute to activities such as regulation, growth, andapoptosis.[7]
Immune responses
editAn example of how glycolipids function within the body is the interaction betweenleukocytesandendothelial cellsduring inflammation.Selectins,a class oflectinsfound on the surface of leukocytes and endothelial cells bind to the carbohydrates attached to glycolipids to initiate the immune response. This binding causes leukocytes to leave circulation and congregate near the site of inflammation. This is the initial binding mechanism, which is followed by the expression ofintegrinswhich form stronger bonds and allow leukocytes to migrate toward the site of inflammation.[8]Glycolipids are also responsible for other responses, notably the recognition of host cells by viruses.[9]
Blood types
editBlood typesare an example of how glycolipids on cell membranes mediate cell interactions with the surrounding environment. The four main human blood types (A, B, AB, O) are determined by theoligosaccharideattached to a specific glycolipid on the surface ofred blood cells,which acts as anantigen.The unmodified antigen, called the H antigen, is the characteristic of type O, and is present on red blood cells of all blood types. Blood type A has anN-acetylgalactosamineadded as the main determining structure, type B has agalactose,and type AB has all three of these antigens. Antigens which are not present in an individual's blood will cause antibodies to be produced, which will bind to the foreign glycolipids. For this reason, people with blood type AB can receive transfusions from all blood types (the universal acceptor), and people with blood type O can act as donors to all blood types (the universal donor).[10]
Types of glycolipids
edit- Glycoglycerolipids: a sub-group of glycolipids characterized by an acetylated or non-acetylated glycerol with at least one fatty acid as the lipid complex. Glyceroglycolipids are often associated withphotosyntheticmembranes and their functions. The subcategories of glyceroglycolipids depend on the carbohydrate attached.[11]
- Galactolipids:defined by a galactose sugar attached to a glycerol lipid molecule. They are found in chloroplast membranes and are associated with photosynthetic properties.[11]
- Sulfolipids:have a sulfur-containing functional group in the sugar moiety attached to a lipid. An important group is thesulfoquinovosyl diacylglycerolswhich are associated with thesulfur cyclein plants.[12]
- Glycosphingolipids:a sub-group of glycolipids based onsphingolipids.Glycosphingolipids are mostly located innervous tissueand are responsible for cell signaling.[13]
- Cerebrosides:a group glycosphingolipids involved in nerve cell membranes.[14]
- Galactocerebrosides:a type of cerebroseide with galactose as the saccharide moiety
- Glucocerebrosides:a type of cerebroside with glucose as the saccharide moiety; often found in non-neural tissue.
- Sulfatides:a class of glycolipids containing a sulfate group in the carbohydrate with aceramidelipid backbone. They are involved in numerous biological functions ranging from immune response to nervous system signaling.
- Gangliosides:the most complex animal glycolipids. They contain negatively charged oligosacchrides with one or moresialic acidresidues; more than 200[15]different gangliosides have been identified. They are most abundant in nerve cells.
- Globosides:glycosphingolipids with more than one sugar as part of the carbohydrate complex. They have a variety of functions; failure to degrade these molecules leads toFabry disease.
- Glycophosphosphingolipids: complex glycophospholipids from fungi, yeasts, and plants, where they were originally called "phytoglycolipids". They may be as complicated a set of compounds as the negatively charged gangliosides in animals.
- Cerebrosides:a group glycosphingolipids involved in nerve cell membranes.[14]
- Glycophosphatidylinositols:a sub-group of glycolipids defined by a phosphatidylinositol lipid moiety bound to a carbohydrate complex. They can be bound to the C-terminus of a protein and have various functions associated with the different proteins they can be bound to.[16]
See also
editReferences
edit- ^Voet D, Voet J, Pratt C (2013).Fundamentals of Biochemistry Life at the Molecular Level(Fourth ed.). Hoboken, NJ: John Wiley & Sons, Inc.ISBN9781118129180.
- ^ab"Glycolipids".nature.Nature Publishing Group.Retrieved1 November2015.
- ^Aureli M, Grassi S, Prioni S, Sonnino S, Prinetti A (August 2015). "Lipid membrane domains in the brain".Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.1851(8):1006–16.doi:10.1016/j.bbalip.2015.02.001.PMID25677824.
- ^Williams GJ, Thorson JS (2009). "Natural product glycosyltransferases: properties and applications".Advances in Enzymology.Advances in Enzymology - and Related Areas of Molecular Biology. Vol. 76. pp.55–119.doi:10.1002/9780470392881.ch2.ISBN9780470392881.PMID18990828.
- ^Sinnott ML (November 1990). "Catalytic mechanism of enzymic glycosyl transfer".Chemical Reviews.90(7):1171–1202.doi:10.1021/cr00105a006.
- ^Sandhoff K (1974)."Sphingolipidoses".Journal of Clinical Pathology.8(12):94–105.doi:10.1136/jcp.s3-8.1.94.PMC1347206.PMID4157247.
- ^Schnaar RL (June 2004). "Glycolipid-mediated cell-cell recognition in inflammation and nerve regeneration".Archives of Biochemistry and Biophysics.426(2):163–72.doi:10.1016/j.abb.2004.02.019.PMID15158667.
- ^Cooper GM (2000)."Cell-Cell Interactions".The Cell: A Molecular Approach(2nd ed.). Sunderland (MA): Sinauer Associates.
- ^Wang B, Boons GJ (9 September 2011).Carbohydrate Recognition: Biological Problems, Methods, and Applications.John Wiley & Sons. p. 66.ISBN9781118017579.
- ^Erb IH (May 1940)."Blood Group Classification: A Plea for Uniformity".Canadian Medical Association Journal.42(5):418–21.PMC537907.PMID20321693.
- ^abNeufeld EF, Hall CW (January 1964). "Formation of galactolipids by chloroplasts".Biochemical and Biophysical Research Communications.14(6):503–8.doi:10.1016/0006-291X(64)90259-1.PMID5836548.
- ^Harwood JL, Nicholls RG (April 1979). "The plant sulpholipid-- a major component of the sulphur cycle".Biochemical Society Transactions.7(2):440–7.doi:10.1042/bst0070440.PMID428677.
- ^Hakomori S,Igarashi Y (December 1995)."Functional role of glycosphingolipids in cell recognition and signaling".Journal of Biochemistry.118(6):1091–103.doi:10.1093/oxfordjournals.jbchem.a124992.PMID8720120.
- ^Jurevics H, Hostettler J, Muse ED, Sammond DW, Matsushima GK, Toews AD, Morell P (May 2001)."Cerebroside synthesis as a measure of the rate of remyelination following cuprizone-induced demyelination in brain".Journal of Neurochemistry.77(4):1067–76.doi:10.1046/j.1471-4159.2001.00310.x.PMID11359872.
- ^Ariga T, McDonald MP, Yu RK (June 2008)."Role of ganglioside metabolism in the pathogenesis of Alzheimer's disease--a review".Journal of Lipid Research.49(6):1157–75.doi:10.1194/jlr.R800007-JLR200.PMC2386904.PMID18334715.
- ^Paulick MG, Bertozzi CR (July 2008)."The glycosylphosphatidylinositol anchor: a complex membrane-anchoring structure for proteins".Biochemistry.47(27):6991–7000.doi:10.1021/bi8006324.PMC2663890.PMID18557633.
External links
edit- Glycolipidsat the U.S. National Library of MedicineMedical Subject Headings(MeSH)