HMX,also calledoctogen,is a powerful and relatively insensitivenitroaminehigh explosivechemically related toRDX.The compound's name is the subject of much speculation, having been variously listed asHigh Melting Explosive,High-velocity Military Explosive,orHigh-Molecular-weight RDX.[1]
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,5,7-Tetranitro-1,3,5,7-tetrazocane | |
| Other names
Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.018.418 |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C4H8N8O8 | |
| Molar mass | 296.155 g/mol |
| Density | 1.91 g/cm3,solid |
| Melting point | 276 to 286 °C (529 to 547 °F; 549 to 559 K) |
| Explosive data | |
| Shock sensitivity | Low |
| Friction sensitivity | Low |
| Detonation velocity | 9100 m/s |
| RE factor | 1.70 |
| Hazards | |
| Occupational safety and health(OHS/OSH): | |
Main hazards
|
Explosive |
| GHSlabelling: | |
 
| |
| Danger | |
| H201,H205,H241,H301,H304,H311,H319 | |
| P210,P250,P280,P370+P380,P372,P373 | |
| NFPA 704(fire diamond) | |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
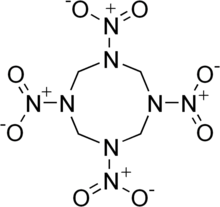
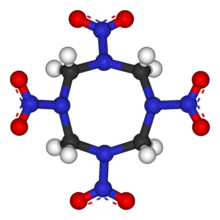
The molecular structure of HMX consists of an eight-membered ring of alternating carbon and nitrogen atoms, with a nitro group attached to each nitrogen atom. Because of its high mass-specificenthalpy of formation,it is one of the most potent chemical explosives manufactured, although a number of newer ones, includingHNIWandONC,are more powerful.
Synthesis
editHMX is more complicated to manufacture than most explosives, and this confines it to specialist applications. It andRDXare both produced by theBachmann process—nitration ofhexamineusing a mixture ofammonium nitrateandnitric acidin a mixture ofacetic acidandacetic anhydrideas solvent—with the major product determined by the specific reaction conditions.[2]
Applications
editAlso known as cyclotetramethylene-tetranitramine, tetrahexamine tetranitramine, or octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine, HMX was first made in 1930. In 1949 it was discovered that HMX can be prepared bynitrolysisof RDX. Nitrolysis of RDX is performed by dissolving RDX in a 55%HNO3solution, followed by placing the solution on a steambath for about six hours.[3]HMX is used almost exclusively in military applications, including as the detonator innuclear weapons,in the form ofpolymer-bonded explosive,and as a solid-rocketpropellant.
HMX is used in melt-castable explosives when mixed withTNT,which as a class are referred to as "octols".Additionally,polymer-bonded explosivecompositions containing HMX are used in the manufacture of missilewarheadsand armor-piercingshaped charges.
HMX is also used in the process ofperforating the steel casing in oil and gas wells.The HMX is built into a shaped charge that is detonated within the wellbore to punch a hole through the steel casing and surrounding cement out into the hydrocarbon-bearing formations. The pathway that is created allows formation fluids to flow into the wellbore and onward to the surface.[4][5]
TheHayabusa2space probe used HMX to excavate a hole in anasteroidin order to access material that had not been exposed to thesolar wind.[6]
Ongoing research aims to reduce its sensitivity and improve some manufacturing properties.[7][8]
Health and environmental fate
editAnalytical methods
editHMX enters the environment through air, water, and soil because it is widely used in military and civil applications. At present, reverse-phase HPLC and more sensitive LC-MS methods have been developed to accurately quantify the concentration of HMX in a variety of matrices in environmental assessments.[9][10]
Toxicity
editAt present, the information needed to determine if HMX causes cancer is insufficient. Due to the lack of information, EPA has determined that HMX is not classifiable as to its human carcinogenicity.[11]
The available data on the effects on human health of exposure to HMX are limited. HMX causes CNS effects similar to those of RDX, but at considerably higher doses. In one study, volunteers submitted topatch testing,which produced skin irritation. Another study of a cohort of 93 workers at an ammunition plant found no hematological, hepatic, autoimmune, or renal diseases. However, the study did not quantify the levels of exposure to HMX.
HMX exposure has been investigated in several studies on animals. Overall, the toxicity appears to be quite low. HMX is poorly absorbed by ingestion. When applied to the dermis, it induces mild skin irritation but not delayed contact sensitization. Various acute and subchronic neurobehavioral effects have been reported in rabbits and rodents, including ataxia, sedation, hyperkinesia, and convulsions. The chronic effects of HMX that have been documented through animal studies include decreased hemoglobin, increased serum alkaline phosphatase, and decreased albumin. Pathological changes were also observed in the animals' livers and kidneys.
Gas exchange rate was used as an indicator of chemical stress in Northern bobwhite quail (Colinus virginianus) eggs, and no evidence of alterations in metabolic rates associated with HMX exposure was observed.[12]No data are available concerning the possible reproductive, developmental, or carcinogenic effects of HMX.[2][13]HMX is considered less toxic thanTNTorRDX.[14]Remediating HMX-contaminated water supplies has proven to be successful.[15]
Biodegradation
editBoth wild and transgenic plants canphytoremediateexplosives from soil and water.[16]
See also
editNotes
edit- ^Cooper, Paul W.,Explosives Engineering,New York: Wiley-VCH, 1996.ISBN0-471-18636-8
- ^abJohn Pike (1996-06-19)."Nitramine Explosives".Globalsecurity.org.Retrieved2012-05-24.
- ^WE Bachmann, JC Sheehan (1949). "A New Method of Preparing the High Explosive RDX1".Journal of the American Chemical Society,1949 (5):1842–1845.
- ^Hansen, Brad (11 March 2013),"Technical Presentation Session 3: Drilling and Completion Casing Perforating Overview"(PDF),Casing Perforation Overview,EPA's Study of Hydraulic Fracturing and Its Potential Impact on Drinking Water Resources, U.S. Environmental Protection Agency
- ^Liu, He; Wang, Feng; Weng, Yucai; Gao, Yang; Cheng, Jianlong (December 2014)."Oil well perforation technology: Status and prospects".Petroleum Exploration and Development.41(6): 798–804.Bibcode:2014PEDO...41..798L.doi:10.1016/S1876-3804(14)60096-3.
- ^Saiki, Takanao; Sawada, Hirotaka; Okamoto, Chisato; Yano, Hajime; Takagi, Yasuhiko; Akahoshi, Yasuhiro; Yoshikawa, Makoto (2013). "Small carry-on impactor of Hayabusa2 mission".Acta Astronautica.84:227–236.Bibcode:2013AcAau..84..227S.doi:10.1016/j.actaastro.2012.11.010.
- ^Kosareva, Ekaterina K.; Zharkov, Mikhail N.; Meerov, Dmitry B.; Gainutdinov, Radmir V.; Fomenkov, Igor V.; Zlotin, Sergei G.; Pivkina, Alla N.; Kuchurov, Ilya V.; Muravyev, Nikita V. (January 2022)."HMX surface modification with polymers via sc-CO2 antisolvent process: A way to safe and easy-to-handle energetic materials".Chemical Engineering Journal.428:131363.doi:10.1016/j.cej.2021.131363.
- ^Lin, Congmei; Zeng, Chengcheng; Wen, Yushi; Gong, Feiyan; He, Guansong; Li, Yubin; Yang, Zhijian; Ding, Ling; Li, Jiang; Guo, Shaoyun (2020-01-22)."Litchi-like Core–Shell HMX@HPW@PDA Microparticles for Polymer-Bonded Energetic Composites with Low Sensitivity and High Mechanical Properties".ACS Applied Materials & Interfaces.12(3): 4002–4013.doi:10.1021/acsami.9b20323.ISSN1944-8244.PMID31874021.S2CID209473864.
- ^Liu, Jun; Severt, Scott A.; Pan, Xiaoping; Smith, Philip N.; McMurry, Scott T.; Cobb, George P. (2007-02-15). "Development of an extraction and cleanup procedure for a liquid chromatographic–mass spectrometric method to analyze octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine in eggs".Talanta.71(2): 627–631.doi:10.1016/j.talanta.2006.05.007.PMID19071351.
- ^Pan, Xiaoping; Zhang, Baohong; Tian, Kang; Jones, Lindsey E.; Liu, Jun; Anderson, Todd A.; Wang, Jia-Sheng; Cobb, George P. (2006-07-30). "Liquid chromatography/electrospray ionization tandem mass spectrometry analysis of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX)".Rapid Communications in Mass Spectrometry.20(14): 2222–2226.Bibcode:2006RCMS...20.2222P.doi:10.1002/rcm.2576.ISSN1097-0231.PMID1679187.
- ^"Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetr... (HMX) (CASRN 2691-41-0) | IRIS | US EPA." EPA. Environmental Protection Agency, n.d. Web. 15 Nov. 2012.[1]
- ^Liu, Jun; Cox, Stephen B.; Beall, Blake; Brunjes, Kristina J.; Pan, Xiaoping; Kendall, Ronald J.; Anderson, Todd A.; McMurry, Scott T.; Cobb, George P. (2008-05-01). "Effects of HMX exposure upon metabolic rate of northern bobwhite quail (Colinus virginianus) in ovo".Chemosphere.71(10): 1945–1949.Bibcode:2008Chmsp..71.1945L.doi:10.1016/j.chemosphere.2007.12.024.ISSN0045-6535.PMID18279915.
- ^"Fact Sheets".Mmr-iagwsp.org.Retrieved2012-05-24.
- ^Daniels, J. I.; Knezovich, J. P. (December 1994)."Information Bridge: DOE Scientific and Technical Information - Sponsored by OSTI"(PDF).Osti.gov.Retrieved2012-05-24.
- ^Newell, Charles. "Treatment of RDX & HMX Plumes Using Mulch Biowalls." ESTCP Project ER-0426. 2008.
- ^Panz K; Miksch K (December 2012). "Phytoremediation of explosives (TNT, RDX, HMX) by wild-type and transgenic plants".Journal of Environmental Management.113:85–92.doi:10.1016/j.jenvman.2012.08.016.PMID22996005.
References
edit- Cooper, Paul W. (1996).Explosives Engineering.New York: Wiley-VCH.ISBN978-0-471-18636-6.OCLC34409473.Retrieved9 June2014.
- Urbanski, Tadeusz (1967).Chemistry and Technology of Explosives.Vol. III. Warszawa: Polish Scientific Publishers.
