Icaridin,also known aspicaridin,is aninsect repellentwhich can be used directly on skin or clothing.[1]It has broad efficacy against various arthropods such as mosquitos, ticks, gnats, flies and fleas, and is almost colorless and odorless. A study performed in 2010 showed that picaridin spray and cream at the 20% concentration provided 12 hours of protection against ticks.[2]UnlikeDEET,icaridin does not dissolve plastics, synthetics or sealants,[3]is odorless and non-greasy[4]and presents a lower risk of toxicity when used with sunscreen, as it may reduce skin absorption of both compounds.[5]
| |||
| Names | |||
|---|---|---|---|
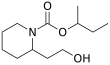
| Preferred IUPAC name
Butan-2-yl 2-(2-hydroxyethyl)piperidine-1-carboxylate | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.102.177 | ||
PubChemCID
|
|||
| UNII | |||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| C12H23NO3 | |||
| Molar mass | 229.320g·mol−1 | ||
| Appearance | colorless liquid | ||
| Odor | odorless | ||
| Density | 1.07 g/cm3 | ||
| Melting point | −170 °C (−274 °F; 103 K) | ||
| Boiling point | 296 °C (565 °F; 569 K) | ||
| 0.82 g/100 mL | |||
| Solubility | 752 g/100mL (acetone) | ||
Refractive index(nD)
|
1.4717 | ||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
The namepicaridinwas proposed as anInternational Nonproprietary Name(INN) to theWorld Health Organization(WHO), but the official name that has been approved by theWHOisicaridin.The chemical is part of thepiperidinefamily,[1]along with many pharmaceuticals andalkaloidssuch aspiperine,which givesblack pepperits spicy taste.
Trade names includeBayrepelandSaltidinamong others. The compound was developed by the German chemical companyBayerin the 1980s[6]and was given the nameBayrepel.In 2005,LanxessAG and its subsidiary Saltigo GmbH were spun off from Bayer[7]and the product was renamedSaltidinin 2008.[8]
Having been sold in Europe (where it is the best-selling insect repellent) since 1998,[9]on 23 July 2020, icaridin was approved again by the EU Commission for use in repellent products. The approval entered into force on 1 February 2022 and is valid for ten years.[10]
Effectiveness
editIcaridin andDEETare the most effective insect repellents available. A 2018 systematic review found no consistent performance difference between icaridin and DEET infield studiesand concluded that they are equally preferred mosquito repellents, noting that 50% DEET offers longer protection but is not available in some countries.[11]
Icaridin has been reported to be as effective as DEET at a 20% concentration without the irritation associated with DEET.[12][13]According to the WHO, icaridin “demonstrates excellent repellent properties comparable to, and often superior to, those of the standard DEET.”
Icaridin-based products have been evaluated byConsumer Reportsin 2016 as among the most effective insect repellents when used at a 20% concentration.[14]Icaridin was earlier reported to be effective byConsumer Reports(7% solution)[15]and the Australian Army (20% solution).[16]Consumer Reportsretests in 2006 gave as result that a 7% solution of icaridin offered little or no protection againstAedesmosquitoes (vectorofdengue fever) and a protection time of about 2.5 hours againstCulex(vector of West Nile virus), while a 15% solution was good for about one hour againstAedesand 4.8 hours againstCulex.[17]
The United StatesCenters for Disease Control and Preventionrecommends using repellents based on icaridin, DEET,ethyl butylacetylaminopropionate(IR3535), or oil oflemon eucalyptus(containingp-menthane-3,8-diol,PMD) for effective protection againstmosquitoesthat carry theWest Nile virus,eastern equine encephalitisand other illnesses.[18]
Adverse effects
editIcaridin can cause mild to moderate eye irritation on contact and is slightly toxic if ingested.[19]
Environmental impact
editA 2018 study found that a commercial repellent product containing 20% icaridin, in what the authors described as "conservative exposure doses", is highly toxic to larval salamanders, a major predator of mosquito larvae.[20]The study observed high larval salamander mortality occurring delayed after the four days of exposure. Because the widely used LC50 test for assessing a chemical's environmental toxicity is based on mortality within four days, the authors suggested that icaridin would be incorrectly deemed as "safe" under the test protocol.[21]However, icaridin was also non-toxic in a 21-day reproduction test on the water fleaDaphnia magna[22]and a 32-day early life-stage test in zebrafish.[23]
Since only the icaridin content of the tested repellent product is known, the observed effects cannot be readily attributed to icaridin. Furthermore, the effects of the repellent product showed no dose-response relationship, i.e., there was neither an increase of the magnitude or severity of the observed effects (mortality, tail deformation), nor did the effects occur at earlier time points. The study has been regarded as invalid by the Danish Environmental Protection Agency,[24][25]which has evaluated icaridin prior to its approval under the EU Biocidal Product Regulation. The reasons for rejection were the testing of a mixture of undisclosed composition, the use of a non-standard test organism, the lack of analytical verification of actual test concentrations, and the fact that the test solution was never renewed with the 25 days of study duration.
Mechanism of action
editIn 2014, a potential odorant receptor for icaridin (and DEET), CquiOR136•CquiOrco, was suggested forCulex quinquefasciatusmosquito.[26]
Recent crystal and solution studies showed that icaridin binds toAnopheles gambiaeodorant binding protein 1 (AgamOBP1). The crystal structure of AgamOBP1•icaridin complex (PDB:5EL2) revealed that icaridin binds to the DEET-binding site in two distinct orientations and also to a second binding site (sIC-binding site) located at the C-terminal region of the AgamOBP1.[27]
Research onAnopheles coluzziimosquitoes suggests icaridin does not strongly activate their olfactory receptor neurons, but instead reduces the volatility of the odorants with which it is mixed.[28]By reducing their volatility, icaridin effectively "masks" odorants attractive to mosquitoes on the skin, preventing them from reaching the olfactory receptors to some extent.[28]
Chemistry
editIcaridin contains twostereocenters:one where the hydroxyethyl chain attaches to the ring, and one where thesec-butylattaches to the oxygen of thecarbamate.The commercial material contains a mixture of all four stereoisomers.
Commercial products
editCommercial products containing icaridin include Cutter Advanced, Muskol, Repeltec,[29]Skin So Soft Bug Guard Plus, Sawyer Picaridin Insect Repellent, Off! FamilyCare, Autan, Smidge, PiActive and MOK.O.[30]
See also
edit- DEET
- Ethyl butylacetylaminopropionate(IR3535)
- Permethrin,apyrethroidinsecticidethat can be applied to clothing to help prevent bites
- p-Menthane-3,8-diol(PMD)
- SS220,another substituted-piperidine insect repellent
References
edit- ^ab"Picaridin".npic.orst.edu.Retrieved2020-03-29.
- ^Carroll, Scott P. (5 April 2010).Efficacy Test of KBR 3023 (Picaridin; Icaridin) - Based Personal Insect Repellents (20% Cream and 20% Spray) with Ticks Under Laboratory Conditions(PDF)(Report). LANXESS Corporation. p. 9.Retrieved8 August2024.
- ^Picaridin.Archived fromthe originalon August 9, 2011.
- ^"Picaridin vs DEET: Which Is the Best Insect Repellent?".Appalachian Mountain Club. 4 August 2023.Retrieved7 August2023.
- ^Rodriguez J, Maibach HI (2016-01-02)."Percutaneous penetration and pharmacodynamics: Wash-in and wash-off of sunscreen and insect repellent".Journal of Dermatological Treatment.27(1):11–18.doi:10.3109/09546634.2015.1050350.ISSN0954-6634.PMID26811157.S2CID40319483.
- ^"Picaridin Technical Fact Sheet".National Pesticide information Center.
- ^"Bayer Completes Spin Off of Lanxess AG".31 January 2005.
- ^Saltigo renames insect repellant,Chemical & Engineering News
- ^"Icaridin - an overview".ScienceDirect.Retrieved29 December2023.
- ^"Commission Implementing Regulation (EU) 2020/1086 of 23 July 2020 approving icaridin as an existing active substance for use in biocidal products of product-type 19".Retrieved31 August2020.
- ^Goodyer, Larry; Schofield, Steven (2018-05-01)."Mosquito repellents for the traveller: does picaridin provide longer protection than DEET?".Journal of Travel Medicine.25(Suppl_1):S10 –S15.doi:10.1093/jtm/tay005.ISSN1708-8305.PMID29718433.
- ^Journal of Drugs in Dermatology (Jan-Feb 2004)http://jddonline.com/articles/dermatology/S1545961604P0059X/1
- ^Van Roey K, Sokny M, Denis L, Van den Broeck N, Heng S, Siv S, et al. (December 2014)."Field evaluation of picaridin repellents reveals differences in repellent sensitivity between Southeast Asian vectors of malaria and arboviruses".PLOS Neglected Tropical Diseases.8(12): e3326.doi:10.1371/journal.pntd.0003326.PMC4270489.PMID25522134.
- ^"Mosquito Repellents That Best Protect Against Zika".Consumer Reports,April, 2016. 30 May 2018.
- ^- Consumer Reports Confirms Effectiveness Of New Alternative To Deet(link recreated from Wayback Machine Internet Archive - 19 May 2019)
- ^Frances SP, Waterson DG, Beebe NW, Cooper RD (May 2004)."Field evaluation of repellent formulations containing deet and picaridin against mosquitoes in Northern Territory, Australia".Journal of Medical Entomology.41(3):414–417.doi:10.1603/0022-2585-41.3.414.PMID15185943.
- ^"Insect repellents: which keep bugs at bay?"Consumer Reports,June 2006, vol 71 (issue 6), p. 6.
- ^"Traveler's Health: Avoid bug bites".Centers for Disease Control and Prevention.
- ^"Picaridin Technical Fact Sheet".National Pesticide Information Center. March 2009.Retrieved6 August2023.
- ^Almeida RM, Han BA, Reisinger AJ, Kagemann C, Rosi EJ (October 2018)."High mortality in aquatic predators of mosquito larvae caused by exposure to insect repellent".Biology Letters.14(10): 20180526.doi:10.1098/rsbl.2018.0526.PMC6227861.PMID30381452.
- ^"Widely used mosquito repellent proves lethal to larval salamanders".Science News.ScienceDaily. Cary Institute of Ecosystem Studies. 31 October 2018.Retrieved12 December2018.
- ^"ECHA - Information on biocides".pp.231–245.Retrieved31 August2020.
- ^"ECHA - Information on biocides".pp.220–229.Retrieved31 August2020.
- ^"ECHA - Information on biocides".echa.europa.eu.p. 3.Retrieved2020-08-31.
- ^"Opinion on the application for approval of the active substance Icaridin, Product type: 19".10 December 2019.Retrieved31 August2020.
- ^Xu P, Choo YM, De La Rosa A, Leal WS (November 2014)."Mosquito odorant receptor for DEET and methyl jasmonate".Proceedings of the National Academy of Sciences of the United States of America.111(46):16592–16597.Bibcode:2014PNAS..11116592X.doi:10.1073/pnas.1417244111.PMC4246313.PMID25349401.
- ^Drakou CE, Tsitsanou KE, Potamitis C, Fessas D, Zervou M, Zographos SE (January 2017)."The crystal structure of the AgamOBP1•Icaridin complex reveals alternative binding modes and stereo-selective repellent recognition".Cellular and Molecular Life Sciences.74(2):319–338.doi:10.1007/s00018-016-2335-6.PMC11107575.PMID27535661.S2CID12211128.
- ^abAfify A, Betz JF, Riabinina O, Lahondère C, Potter CJ (November 2019)."Commonly Used Insect Repellents Hide Human Odors from Anopheles Mosquitoes".Current Biology.29(21): 3669–3680.e5.Bibcode:2019CBio...29E3669A.doi:10.1016/j.cub.2019.09.007.PMC6832857.PMID31630950.
- ^"Insect Repellent Solutions | Repeltec | AFFIX Labs | Finland".Archived fromthe originalon 2022-08-16.Retrieved2024-08-08.
- ^Cha, Ariana Eunjung. "Zika virus FAQ: What is it, and what are the risks as it spreads?The Washington Post.January 21, 2016.