Maltose(/ˈmɔːltoʊs/[2]or/ˈmɔːltoʊz/[3]), also known asmaltobioseormalt sugar,is adisaccharideformed from two units ofglucosejoined with an α(1→4)bond.In theisomerisomaltose,the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of theamylosehomologous series,the key structural motif ofstarch.Whenbeta-amylasebreaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found ingerminatingseeds, which is why it was named aftermalt.[4]Unlikesucrose,it is areducing sugar.[5]
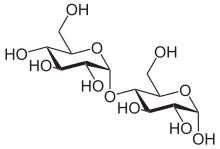 α-Maltose
| |
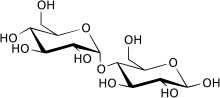 β-Maltose
| |
| Names | |
|---|---|
| IUPAC name
4-O-α-D-Glucopyranosyl-D-glucose
| |
| Systematic IUPAC name
(3R,4R,5S,6R)-6-(hydroxymethyl)-5-{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxane-2,3,4-triol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.651 |
| EC Number |
|
| KEGG | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties[1] | |
| C12H22O11 | |
| Molar mass | 342.297g·mol−1 |
| Appearance | White powder or crystals |
| Density | 1.54 g/cm3 |
| Melting point | 160 to 165 °C (320 to 329 °F; 433 to 438 K) (anhydrous) 102–103 °C (monohydrate) |
| 1.080 g/mL (20 °C) | |
Chiral rotation([α]D)
|
+140.7° (H2O,c= 10) |
| Hazards | |
| Safety data sheet(SDS) | External MSDS |
| Related compounds | |
Related
|
Sucrose Lactose Trehalose Cellobiose |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |

History
editMaltose was discovered byAugustin-Pierre Dubrunfaut,although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewerCornelius O'Sullivan.[5][6]Its name comes frommalt,combined with the suffix '-ose' which is used in names of sugars.[4]
Structure and nomenclature
editCarbohydrates are generally divided intomonosaccharides,oligosaccharides,andpolysaccharidesdepending on the number of sugar subunits. Maltose, with two sugar units, is a disaccharide, which falls under oligosaccharides. Glucose is ahexose:a monosaccharide containing six carbon atoms. The two glucose units are in thepyranoseform and are joined by anO-glycosidic bond,with the first carbon (C1) of the firstglucoselinked to the fourth carbon (C4) of the secondglucose,indicated as (1→4). The link is characterized as α because the glycosidic bond to the anomeric carbon (C1) is in the opposite plane from theCH
2OHsubstituentin the same ring (C6of the first glucose). If the glycosidic bond to the anomeric carbon (C1) were in the same plane as theCH
2OHsubstituent, it would be classified as aβ(1→4)bond, and the resulting molecule would becellobiose.The anomeric carbon (C1) of the second glucose molecule, which is not involved in a glycosidic bond, could be either an α- or β-anomer depending on the bond direction of the attached hydroxyl group relative to theCH
2OHsubstituent of the same ring, resulting in either α-maltose or β-maltose.[citation needed]
Anisomerof maltose isisomaltose.This is similar to maltose but instead of a bond in the α(1→4) position, it is in the α(1→6) position, the same bond that is found at the branch points ofglycogenandamylopectin.[citation needed]
Properties
editLike glucose, maltose is areducing sugar,because the ring of one of the two glucose units can open to present a freealdehydegroup; the other one cannot because of the nature of the glycosidic bond. Maltose can be broken down to glucose by themaltaseenzyme, which catalyses the hydrolysis of the glycosidic bond.[citation needed]
Maltose in aqueous solution exhibitsmutarotation,because the α and β isomers that are formed by the different conformations of the anomeric carbon have differentspecific rotations,and in aqueous solutions, these two forms are in equilibrium. Maltose can easily be detected by the Woehlk test or Fearon's test on methylamine.[7]
It has a sweet taste, but is only about 30–60% as sweet as sugar, depending on the concentration.[8]A 10% solution of maltose is 35% as sweet as sucrose.[9]
Sources and absorption
editMaltose is amaltcomponent, a substance obtained when the grain is softened in water and germinates. It is also present in highly variable quantities in partially hydrolyzed starch products likemaltodextrin,corn syrupand acid-thinned starch.[10]
Outside of plants, maltose is also (likely) found insugarbag.[11]
In humans, maltose is broken down by various maltase enzymes, providing two glucose molecules that can befurther processed:either broken down to provide energy, or stored as glycogen. The lack of thesucrase-isomaltaseenzyme in humans causessucrose intolerance,but complete maltose intolerance is extremely rare because there are four different maltase enzymes.[12]
References
edit- ^Weast, Robert C., ed. (1981).CRC Handbook of Chemistry and Physics(62nd ed.). Boca Raton, FL: CRC Press. p. C-367.ISBN0-8493-0462-8..
- ^Dictionary Reference:maltose
- ^Cambridge dictionary:maltose
- ^abStoker, H. Stephen (2 January 2015).Organic and Biological Chemistry.Cengage Learning.ISBN9781305686458.
- ^abFruton, Joseph S (1999).Proteins, Enzymes, Genes: The Interplay of Chemistry and Biology.Chelsea, Michigan: Yale University Press. p. 144.ISBN0300153597.Retrieved21 October2017.
- ^O'Sullivan, Cornelius (1872)."XXI.?On the transformation-products of starch".Journal of the Chemical Society.25:579–588.doi:10.1039/JS8722500579.Retrieved11 December2014.
- ^"150 Years Alfred Wöhlk:: Education:: ChemistryViews".6 March 2018.
- ^Belitz, H.-D.; Grosch, Werner; Schieberle, Peter (15 January 2009).Food Chemistry.Springer Science & Business Media. p. 863.ISBN9783540699330.
- ^Spillane, W. J. (17 July 2006).Optimising Sweet Taste in Foods.Woodhead Publishing. p. 271.ISBN9781845691646.
- ^Furia, Thomas E. (2 January 1973).CRC Handbook of Food Additives, Second Edition.CRC Press.ISBN9780849305429.
- ^Heard, Tim (30 October 2015).The Australian Native Bee Book.Sugarbag Bees.ISBN9780646939971.
- ^Whelan, W. J.; Cameron, Margaret P. (16 September 2009).Control of Glycogen Metabolism.John Wiley & Sons. p. 60.ISBN9780470716885.