Mianserin,sold under the brand nameTolvonamong others, is anatypical antidepressantthat is used primarily in the treatment ofdepressioninEuropeand elsewhere in the world.[6]It is atetracyclic antidepressant(TeCA). Mianserin is closely related tomirtazapine,bothchemicallyand in terms of its actions and effects, although there are significant differences between the two drugs (for example, its highernoradrenergicactivity and lower5-HT3 receptorantagonism).[7]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tolvon, others |
| Other names | Mianserin hydrochloride; Org GB 94[1][2] |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokineticdata | |
| Bioavailability | 20–30%[4] |
| Protein binding | 95%[4] |
| Metabolism | Liver(CYP2D6;viaaromatichydroxylation,N-oxidation,N-demethylation)[4] |
| Eliminationhalf-life | 21–61 hours[5] |
| Excretion | Urine:4–7%[4] Feces:14–28%[4] |
| Identifiers | |
| |
| CAS Number |
|
| PubChemCID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.041.884 |
| Chemical and physical data | |
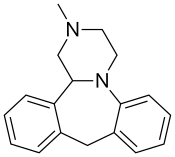
| Formula | C18H20N2 |
| Molar mass | 264.372g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Medical uses
editMianserin at higher doses (30–90 mg/day) is used for the treatment ofmajor depressive disorder.[6]
It can also be used at lower doses (around 10 mg/day) to treat insomnia.[8][9]
Contraindications
editIt should not be given, except if based on clinical need and under strict medical supervision, to people younger than 18 years old, as it can increase the risk of suicide attempts and suicidal thinking, and it can increase aggressiveness.[6]
While there is no evidence that it can harm a fetus from animal models, there are no data showing it safe for pregnant women to take.[6]
People with severeliver diseaseshould not take mianserin, and it should be used with caution for people withepilepsyor who are at risk for seizures, as it can lower the threshold for seizures. If based on clinical decision, normal precautions should be exercised and the dosages of mianserin and any concurrent therapy kept under review and adjusted as needed.[6]
Side effects
editVery common (incidence > 10%) adverse effects include constipation, dry mouth, and drowsiness at the beginning of treatment.[5][6]
Common (1% < incidence ≤ 10%) adverse effects include drowsiness during maintenance therapy, tremor, headache, dizziness, vertigo, and weakness.[5]
Uncommon (0.1% < incidence ≤ 1%) adverse effects include weight gain.[5]
Withdrawal
editAbrupt or rapid discontinuation of mianserin may provoke awithdrawal,theeffectsof which may includedepression,anxiety,panic attacks,[10]decreasedappetiteoranorexia,insomnia,diarrhea,nauseaandvomiting,andflu-like symptoms, such asallergiesorpruritus,among others.
Overdose
editOverdose of mianserin is known to produce sedation, coma, hypotension or hypertension, tachycardia, and QT interval prolongation.[11]
Interactions
editMianserin may enhance the sedative effects of drugs such asalcohol,anxiolytics, hypnotics, or antipsychotics when co-administered. It may decrease the efficacy ofantiepilepticmedications.
Carbamazepineandphenobarbitalwill cause the body to metabolize mianserin faster and may reduce its effects. There is a risk of dangerously low blood pressure if people take mianserin along withdiazoxide,hydralazine,ornitroprusside.Mianserin can makeantihistaminesandantimuscarinicshave stronger effects. Mianserin should not be taken withapraclonidine,brimonidine,sibutramine,or the combination drug ofartemetherwithlumefantrine.[6]
Pharmacology
editPharmacodynamics
edit| Site | Ki(nM) | Species | Ref |
|---|---|---|---|
| SERT | 4000 | Human | [13] |
| NET | 71 | Human | [13] |
| DAT | 9400 | Human | [13] |
| 5-HT1A | 400–2600 | Human | [14][15] |
| 5-HT1B | 2800+ | Rat | [16] |
| 5-HT1D | 220–400 | Human | [17][18] |
| 5-HT1E | ND | ND | ND |
| 5-HT1F | 13 | Human | [14] |
| 5-HT2A | 1.6–55 | Human | [19][20] |
| 5-HT2B | 1.6–20 | Human | [21][22] |
| 5-HT2C | 0.63–6.5 | Human | [19][23] |
| 5-HT3 | 5.8–300 | Rodent | [24][15] |
| 5-HT4 | ND | ND | ND |
| 5-HT5A | ND | ND | ND |
| 5-HT6 | 55–81 | Human | [25][26] |
| 5-HT7 | 48–56 | Human | [27][28][29] |
| α1 | 34 | Human | [30] |
| α2 | 73 | Human | [30] |
| α2A | 4.8 | Human | [27] |
| α2B | 27 | Human | [31] |
| α2C | 3.8 | Human | [27] |
| D1 | 426–1420 | Human | [15][27] |
| D2 | 2100–2700 | Human | [30][32] |
| D3 | 2840 | Human | [30] |
| D4 | ND | ND | ND |
| D5 | ND | ND | ND |
| H1 | 0.30–1.7 | Human | [33][30][27] |
| H2 | 437 | Human | [34] |
| H3 | 95500 | Human | [34] |
| H4 | 100000+ | Human | [34][35] |
| mACh | 820 | Human | [30] |
| MOR | 21000 | Human | [36] |
| DOR | 30200 | Human | [36] |
| KOR | 530 (EC50) | Human | [36] |
| Values are Ki(nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. | |||
Mianserin appears to exert its effects via antagonism ofhistamineandserotoninreceptors, and inhibition ofnorepinephrinereuptake. More specifically, it is anantagonist/inverse agonistat most or all sites of thehistamineH1receptor,serotonin5-HT1D,5-HT1F,5-HT2A,5-HT2B,5-HT2C,5-HT3,5-HT6,and5-HT7receptors,and adrenergicα1-andα2-adrenergic receptors,and additionally anorepinephrinereuptake inhibitor.[37][38]As an H1receptor inverse agonist with highaffinity,mianserin has strongantihistamineeffects (e.g.,sedation). Conversely, it has low affinity for themuscarinic acetylcholine receptors,and hence lacksanticholinergicproperties.[30]Mianserin has been found to be a low affinity but potentially significantpartial agonistof theκ-opioid receptor(Ki= 1.7 μM;EC50= 0.53 μM),[36]similarly to sometricyclic antidepressants(TCAs).[39]
Blockade of the H1and possibly α1-adrenergic receptors hassedativeeffects,[5]and also antagonism of the 5-HT2Aand α1-adrenergic receptors inhibits activation ofintracellularphospholipase C(PLC), which seems to be a common target for several differentclassesofantidepressants.[40]By antagonizing thesomatodendriticandpresynapticα2-adrenergic receptors, which function predominantly asinhibitoryautoreceptorsandheteroreceptors,mianserin disinhibits the release ofnorepinephrine,dopamine,serotonin,andacetylcholinein various areas of thebrainandbody.
Along with mirtazapine, although to a lesser extent in comparison, mianserin has sometimes been described as anoradrenergic and specific serotonergic antidepressant(NaSSA).[41]However, the actual evidence in support of this label has been regarded as poor.[42]
Pharmacokinetics
editThebioavailabilityof mianserin is 20 to 30%.[4]Itsplasma protein bindingis 95%.[4]Mianserin ismetabolizedin theliverby theCYP2D6enzymeviaN-oxidationandN-demethylation.[4]Itselimination half-lifeis 21 to 61 hours.[4]The drug isexcreted4 to 7% in theurineand 14 to 28% infeces.[4]
Chemistry
editMianserin is a tetracyclicpiperazinoazepine.Mirtazapinewas developed by the same team of organic chemists and differs via addition of a nitrogen atom in one of the rings.[43][44](S)-(+)-Mianserin is approximately 200–300 times more active than itsenantiomer(R)-(−)-mianserin; hence, the activity of mianserin lies in the (S)-(+)isomer.[citation needed]
History
editIt wasdevelopedbut not discovered byOrganon International;the first patents were issued in The Netherlands in 1967, and it was launched in France in 1979 under the brand name Athymil, and soon thereafter in the UK as Norval. Investigators conducting clinical trials in the US submitted fraudulent data, and it was never approved in the US.[45]: 21 [46]: 318
Mianserin was one of the first antidepressants to reach the UK market that was less dangerous than thetricyclic antidepressantsin overdose; as of 2012 it was not prescribed much in the UK.[47]
Society and culture
editGeneric names
editMianserinis theEnglishandGermangeneric nameof the drug and itsINNandBAN,whilemianserin hydrochlorideis itsUSAN,BANM,andJAN.Its generic name inFrenchand itsDCFaremiansérine,inSpanishandItalianand itsDCITaremianserina,and inLatinismianserinum.[48][1][49][2]
Brand names
editMianserin is marketed in many countries mainly under the brand name Tolvon. It is also available throughout the world under a variety of other brand names including Athymil, Bonserin, Bolvidon, Deprevon, Lantanon, Lerivon, Lumin, Miansan, Serelan, Tetramide, and Tolvin among others.[1][2][48]
Availability
editMianserin is not approved for use in theUnited States,but is available in theUnited Kingdomand otherEuropeancountries.[50][51]A mianserin generic drug receivedTGAapproval in May 1996 and is available inAustralia.[52]
Research
editThe use of mianserin to help people with schizophrenia who are being treated with antipsychotics has been studied in clinical trials; the outcome is unclear.[53][54]
References
edit- ^abcIndex Nominum 2000: International Drug Directory.Taylor & Francis. 2000. pp. 689–.ISBN978-3-88763-075-1.
- ^abc"International brands for mianserin".Drugs.com.Retrieved20 August2017.
- ^Anvisa(2023-03-31)."RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial"[Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese).Diário Oficial da União(published 2023-04-04).Archivedfrom the original on 2023-08-03.Retrieved2023-08-16.
- ^abcdefghijTruven Health Analytics, Inc. Drugdex System (Internet) [cited 2013 Sep 29]. Greenwood Village, CO: Thomsen Healthcare; 2013.
- ^abcde"Tolvon Product Information"(PDF).Medicines.AU:Merck Sharp & Dohme. Archived fromthe original(PDF)on 2016-04-02.Retrieved2013-10-05– via GuildLink.
- ^abcdefg"Mianserin 30 mg film-coated tablets".UK Electronic Medicines Compendium. January 2014.Retrieved20 August2017.
- ^"A Comparison of the Physicochemical and Biological Properties of Mirtazapine and Mianserin".Journal of pharmacy and pharmacology.Oxford University Press. April 2011.Retrieved29 January2022.
- ^"Que faire devant une insomnie"[What to do when facing insomnia].Sommeil(in French).Lyon,FR:University of Lyon.
- ^"Traitement des troubles du sommeil"[Treatment of the troubles of sleep].Research gate(in French). Archived fromthe originalon 2019-03-30.
- ^Kuniyoshi M, Arikawa K, Miura C, Inanaga K (June 1989). "Panic anxiety after abrupt discontinuation of mianserin".The Japanese Journal of Psychiatry and Neurology.43(2):155–59.doi:10.1111/j.1440-1819.1989.tb02564.x.PMID2796025.S2CID527031.
- ^Taylor D, Paton C, Kapur S, Taylor D (2012).The Maudsley Prescribing Guidelines in Psychiatry(11th ed.). Chichester, West Sussex: John Wiley & Sons.
- ^Roth BL,Driscol J."PDSP KiDatabase ".Psychoactive Drug Screening Program (PDSP).University of North Carolina at Chapel Hill and the United States National Institute of Mental Health.Retrieved14 August2017.
- ^abcTatsumi M, Groshan K, Blakely RD, Richelson E (December 1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters".European Journal of Pharmacology.340(2–3):249–258.doi:10.1016/s0014-2999(97)01393-9.PMID9537821.
- ^abBoess FG, Martin IL (1994). "Molecular biology of 5-HT receptors".Neuropharmacology.33(3–4):275–317.doi:10.1016/0028-3908(94)90059-0.PMID7984267.S2CID35553281.
- ^abcToll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, et al. (March 1998). "Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications".NIDA Research Monograph.178:440–466.PMID9686407.
- ^Matsumoto I, Combs MR, Jones DJ (February 1992). "Characterization of 5-hydroxytryptamine1B receptors in rat spinal cord via [125I]iodocyanopindolol binding and inhibition of [3H]-5-hydroxytryptamine release".The Journal of Pharmacology and Experimental Therapeutics.260(2):614–626.PMID1738111.
- ^Peroutka SJ, Switzer JA, Hamik A (1989). "Identification of 5-hydroxytryptamine1D binding sites in human brain membranes".Synapse.3(1):61–66.doi:10.1002/syn.890030109.PMID2521959.S2CID23503235.
- ^Waeber C, Schoeffter P, Palacios JM, Hoyer D (June 1988). "Molecular pharmacology of 5-HT1D recognition sites: radioligand binding studies in human, pig and calf brain membranes".Naunyn-Schmiedeberg's Archives of Pharmacology.337(6):595–601.doi:10.1007/bf00175783.PMID2975354.S2CID21344978.
- ^abMillan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, et al. (February 2000). "Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states".Synapse.35(2):79–95.doi:10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X.PMID10611634.S2CID20221398.
- ^Elliott JM, Kent A (July 1989). "Comparison of [125I]iodolysergic acid diethylamide binding in human frontal cortex and platelet tissue".Journal of Neurochemistry.53(1):191–196.doi:10.1111/j.1471-4159.1989.tb07313.x.PMID2723656.S2CID25820829.
- ^Bonhaus DW, Flippin LA, Greenhouse RJ, Jaime S, Rocha C, Dawson M, et al. (July 1999)."RS-127445: a selective, high affinity, orally bioavailable 5-HT2B receptor antagonist".British Journal of Pharmacology.127(5):1075–1082.doi:10.1038/sj.bjp.0702632.PMC1566110.PMID10455251.
- ^Bonhaus DW, Bach C, DeSouza A, Salazar FH, Matsuoka BD, Zuppan P, et al. (June 1995)."The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors".British Journal of Pharmacology.115(4):622–628.doi:10.1111/j.1476-5381.1995.tb14977.x.PMC1908489.PMID7582481.
- ^Wainscott DB, Lucaites VL, Kursar JD, Baez M, Nelson DL (February 1996). "Pharmacologic characterization of the human 5-hydroxytryptamine2B receptor: evidence for species differences".The Journal of Pharmacology and Experimental Therapeutics.276(2):720–727.PMID8632342.
- ^Nelson DR, Thomas DR (May 1989). "[3H]-BRL 43694 (Granisetron), a specific ligand for 5-HT3 binding sites in rat brain cortical membranes".Biochemical Pharmacology.38(10):1693–1695.doi:10.1016/0006-2952(89)90319-5.PMID2543418.
- ^Kohen R, Metcalf MA, Khan N, Druck T, Huebner K, Lachowicz JE, et al. (January 1996). "Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor".Journal of Neurochemistry.66(1):47–56.doi:10.1046/j.1471-4159.1996.66010047.x.PMID8522988.S2CID35874409.
- ^Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, Medhurst AD (December 2003). "Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling".Molecular Pharmacology.64(6):1295–1308.doi:10.1124/mol.64.6.1295.PMID14645659.
- ^abcdeFernández J, Alonso JM, Andrés JI, Cid JM, Díaz A, Iturrino L, et al. (March 2005). "Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents".Journal of Medicinal Chemistry.48(6):1709–1712.doi:10.1021/jm049632c.PMID15771415.
- ^Jasper JR, Kosaka A, To ZP, Chang DJ, Eglen RM (September 1997)."Cloning, expression and pharmacology of a truncated splice variant of the human 5-HT7 receptor (h5-HT7b)".British Journal of Pharmacology.122(1):126–132.doi:10.1038/sj.bjp.0701336.PMC1564895.PMID9298538.
- ^Eglen RM, Jasper JR, Chang DJ, Martin GR (April 1997). "The 5-HT7 receptor: orphan found".Trends in Pharmacological Sciences.18(4):104–107.doi:10.1016/s0165-6147(97)01043-2.PMID9149537.
- ^abcdefgRichelson E, Nelson A (July 1984). "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro".The Journal of Pharmacology and Experimental Therapeutics.230(1):94–102.PMID6086881.
- ^Weinshank RL, Zgombick JM, Macchi M, Adham N, Lichtblau H, Branchek TA, Hartig PR (November 1990). "Cloning, expression, and pharmacological characterization of a human alpha 2B-adrenergic receptor".Molecular Pharmacology.38(5):681–688.PMID2172775.
- ^Grandy DK, Marchionni MA, Makam H, Stofko RE, Alfano M, Frothingham L, et al. (December 1989)."Cloning of the cDNA and gene for a human D2 dopamine receptor".Proceedings of the National Academy of Sciences of the United States of America.86(24):9762–9766.Bibcode:1989PNAS...86.9762G.doi:10.1073/pnas.86.24.9762.PMC298581.PMID2532362.
- ^Ghoneim OM, Legere JA, Golbraikh A, Tropsha A, Booth RG (October 2006). "Novel ligands for the human histamine H1 receptor: synthesis, pharmacology, and comparative molecular field analysis studies of 2-dimethylamino-5-(6)-phenyl-1,2,3,4-tetrahydronaphthalenes".Bioorganic & Medicinal Chemistry.14(19):6640–6658.doi:10.1016/j.bmc.2006.05.077.PMID16782354.
- ^abcAppl H, Holzammer T, Dove S, Haen E, Strasser A, Seifert R (February 2012). "Interactions of recombinant human histamine H₁R, H₂R, H₃R, and H₄R receptors with 34 antidepressants and antipsychotics".Naunyn-Schmiedeberg's Archives of Pharmacology.385(2):145–170.doi:10.1007/s00210-011-0704-0.PMID22033803.S2CID14274150.
- ^Nguyen T, Shapiro DA, George SR, Setola V, Lee DK, Cheng R, et al. (March 2001)."Discovery of a novel member of the histamine receptor family".Molecular Pharmacology.59(3):427–433.doi:10.1124/mol.59.3.427.PMID11179435.
- ^abcdOlianas MC, Dedoni S, Onali P (November 2012)."The atypical antidepressant mianserin exhibits agonist activity at κ-opioid receptors".British Journal of Pharmacology.167(6):1329–1341.doi:10.1111/j.1476-5381.2012.02078.x.PMC3504997.PMID22708686.
- ^Leonard B, Richelson H (2000). "Synaptic Effects of Antidepressants: Relationship to Their Therapeutic and Adverse Effects". In Buckley JL, Waddington PF (eds.).Schizophrenia and Mood Disorders: The New Drug Therapies in Clinical Practice.Oxford: Butterworth-Heinemann. pp.67–84.ISBN978-0-7506-4096-1.
- ^Müller G (8 May 2006)."Target Family-directed Masterkeys in Chemogenomics".In Kubinyi H, Müller G, Mannhold R, Folkers G (eds.).Chemogenomics in Drug Discovery: A Medicinal Chemistry Perspective.John Wiley & Sons. p. 25.ISBN978-3-527-60402-9.
- ^Onali P, Dedoni S, Olianas MC (January 2010). "Direct agonist activity of tricyclic antidepressants at distinct opioid receptor subtypes".The Journal of Pharmacology and Experimental Therapeutics.332(1):255–265.doi:10.1124/jpet.109.159939.PMID19828880.S2CID18893305.
- ^Dwivedi Y, Agrawal AK, Rizavi HS, Pandey GN (December 2002). "Antidepressants reduce phosphoinositide-specific phospholipase C (PI-PLC) activity and the mRNA and protein expression of selective PLC beta 1 isozyme in rat brain".Neuropharmacology.43(8):1269–1279.doi:10.1016/S0028-3908(02)00253-8.PMID12527476.S2CID22105260.
- ^Kishi T, Iwata N (February 2014)."Meta-analysis of noradrenergic and specific serotonergic antidepressant use in schizophrenia".The International Journal of Neuropsychopharmacology.17(2):343–354.doi:10.1017/S1461145713000667.PMID23823741.
- ^Gillman PK (March 2006). "A systematic review of the serotonergic effects of mirtazapine in humans: implications for its dual action status".Human Psychopharmacology.21(2):117–125.doi:10.1002/hup.750.PMID16342227.S2CID23442056.
- ^"Mirtazapine label – Australia".GuildLink, Pharmacy Guild of Australia. 27 May 2016. Archived from the original on 21 November 2018.Retrieved21 June2017.
- ^Kelder J, Funke C, De Boer T, Delbressine L, Leysen D, Nickolson V (April 1997)."A comparison of the physicochemical and biological properties of mirtazapine and mianserin".The Journal of Pharmacy and Pharmacology.49(4):403–11.doi:10.1111/j.2042-7158.1997.tb06814.x.PMID9232538.S2CID12270528.
- ^Shorter E (2005).A historical dictionary of psychiatry.Oxford: Oxford Univ. Press.ISBN978-0-19-517668-1.
- ^Stahl SM (2013).Stahl's essential psychopharmacology: neuroscientific basis and practical application(4th ed.). Cambridge: Cambridge University Press.ISBN978-1-10702598-1.
- ^Pratt JP (2012). "29. Affective Disorders". In Walker R, Whittlesea C (eds.).Clinical pharmacy and therapeutics(5th ed.). Edinburgh: Churchill Livingston/Elsevier. p. 472.ISBN978-0-70204293-5.
- ^abElks J (14 November 2014).The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies.Springer. pp. 822–.ISBN978-1-4757-2085-3.
- ^Morton IK, Hall JM (31 October 1999).Concise Dictionary of Pharmacological Agents: Properties and Synonyms.Springer Science & Business Media. pp. 181–.ISBN978-0-7514-0499-9.
- ^Gelenberg AJ, Schoonover SC (29 June 2013)."Major Psychiatric Disorders: Depression".In Gelenberg AJ, Bassuk EL, Schoonover SC (eds.).The Practitioner's Guide to Psychoactive Drugs.Springer Science & Business Media. pp. 39–.ISBN978-1-4757-1137-0.
- ^Quitkin FM, Taylor BP (24 May 2013)."Antidepressants".In Klein DF, Rowland LP (eds.).Current Psychotherapeutic Drugs.Routledge. pp. 57–.ISBN978-1-135-06284-2.
- ^"Lumin Mianserin hydrochloride product information"(PDF).Medicines.AlphaPharm – via GuildLink.
- ^Terevnikov V, Joffe G, Stenberg JH (May 2015)."Randomized Controlled Trials of Add-On Antidepressants in Schizophrenia".The International Journal of Neuropsychopharmacology.18(9): pyv049.doi:10.1093/ijnp/pyv049.PMC4576515.PMID25991654.
- ^Vernon JA, Grudnikoff E, Seidman AJ, Frazier TW, Vemulapalli MS, Pareek P, et al. (November 2014)."Antidepressants for cognitive impairment in schizophrenia--a systematic review and meta-analysis".Schizophrenia Research.159(2–3):385–394.doi:10.1016/j.schres.2014.08.015.PMC4252251.PMID25240772.
Further reading
edit- Peet M, Behagel H (1978)."Mianserin: a decade of scientific development".British Journal of Clinical Pharmacology.5(Suppl 1):5S –9S.doi:10.1111/j.1365-2125.1978.tb04567.x.PMC1429213.PMID623702.