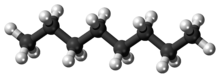
Octaneis ahydrocarbonand analkanewith thechemical formulaC8H18,and the condensed structural formula CH3(CH2)6CH3.Octane has manystructural isomersthat differ by the location of branching in thecarbon chain.One of these isomers,2,2,4-trimethylpentane(commonly called iso-octane), is used as one of the standard values in theoctane ratingscale.
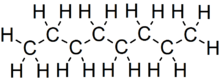
| |
| |
| |
| Names | |
|---|---|
| Systematic IUPAC name
Octane[1] | |
| Other names
n-Octane
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 1696875 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.539 |
| EC Number |
|
| 82412 | |
| KEGG | |
| MeSH | octane |
PubChemCID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1262 |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| CH3(CH2)6CH3 | |
| Molar mass | 114.232g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Gasoline-like[2] |
| Density | 0.703 g/cm3 |
| Melting point | −57.1 to −56.6 °C; −70.9 to −69.8 °F; 216.0 to 216.6 K |
| Boiling point | 125.1 to 126.1 °C; 257.1 to 258.9 °F; 398.2 to 399.2 K |
| 0.007 mg/dm3(at 20 °C) | |
| logP | 4.783 |
| Vapor pressure | 1.47 kPa (at 20.0 °C) |
| 29 nmol/(Pa·kg) | |
| Conjugate acid | Octonium |
| −96.63·10−6cm3/mol | |
Refractive index(nD)
|
1.398 |
| Viscosity |
|
| Thermochemistry | |
| 255.68 J/(K·mol) | |
Std molar
entropy(S⦵298) |
361.20 J/(K·mol) |
Std enthalpy of
formation(ΔfH⦵298) |
−252.1 to −248.5 kJ/mol |
Std enthalpy of
combustion(ΔcH⦵298) |
−5.53 to −5.33 MJ/mol |
| Hazards | |
| GHSlabelling: | |
   
| |
| Danger | |
| H225,H304,H315,H336,H410 | |
| P210,P261,P273,P301+P310,P331 | |
| NFPA 704(fire diamond) | |
| Flash point | 13.0 °C (55.4 °F; 286.1 K) |
| 220.0 °C (428.0 °F; 493.1 K) | |
| Explosive limits | 0.96 – 6.5% |
| Lethal doseor concentration (LD, LC): | |
LDLo(lowest published)
|
428 mg/kg (mouse, intravenous)[4] |
| NIOSH(US health exposure limits): | |
PEL(Permissible)
|
TWA 500 ppm (2350 mg/m3)[2] |
REL(Recommended)
|
TWA 75 ppm (350 mg/m3) C 385 ppm (1800 mg/m3) [15-minute][2] |
IDLH(Immediate danger)
|
1000 ppm[2] |
| Related compounds | |
Related alkanes
|
|
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Octane is a component ofgasolineand petroleum. Understandard temperature and pressure,octane is an odorless, colorless liquid. Like other short-chained alkanes with a low molecular weight, it isvolatile,flammable, and toxic. Octane is 1.2 to 2 times more toxic thanHeptane.[5]
Isomers
editN-octane has 23constitutional isomers.8 of these isomers have onestereocenter;3 of them have two stereocenters.
Achiral Isomers:
- 2-Methylheptane
- 4-Methylheptane
- 3-Ethylhexane
- 2,2-Dimethylhexane
- 2,5-Dimethylhexane
- (meso)-3,4-Dimethylhexane
- 3,3-Dimethylhexane
- 3-Ethyl-2-methylpentane
- 3-Ethyl-3-methylpentane
- 2,2,4-Trimethylpentane(i.e. iso-octane)
- 2,3,3-Trimethylpentane
- 2,3,4-Trimethylpentane
- 2,2,3,3-Tetramethylbutane
Chiral Isomers:
Production and Use
editIn petrochemistry, octanes are not typically differentiated or purified as specific compounds. Octanes are components of particular boiling fractions.[6]
A common route to such fractions is thealkylation reactionbetween iso-butane and 1-butene, which forms iso-octane.[7]
Octane is commonly used as a solvent in paints and adhesives.
References
edit- ^"octane - Compound Summary".PubChem Compound.USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records.Retrieved6 January2012.
- ^abcdNIOSH Pocket Guide to Chemical Hazards."#0470".National Institute for Occupational Safety and Health(NIOSH).
- ^Dymond, J. H.; Oye, H. A. (1994). "Viscosity of Selected Liquidn-Alkanes ".Journal of Physical and Chemical Reference Data.23(1): 41–53.Bibcode:1994JPCRD..23...41D.doi:10.1063/1.555943.ISSN0047-2689.
- ^"Octane".Immediately Dangerous to Life or Health Concentrations (IDLH).National Institute for Occupational Safety and Health(NIOSH).
- ^"1988 OSHA PEL Project - Octane | NIOSH | CDC".www.cdc.gov.2020-02-27.Retrieved2024-04-19.
- ^"Fractionation".www.appliedcontrol.com.Retrieved2024-04-19.
- ^Ross, Julian (January 1986)."Ullmann's Encyclopedia of industrial chemistry".Applied Catalysis.27(2): 403–404.doi:10.1016/s0166-9834(00)82943-7.ISSN0166-9834.
External links
edit- International Chemical Safety Card 0933
- NIOSH Pocket Guide to Chemical Hazards."#0470".National Institute for Occupational Safety and Health(NIOSH).
- Dr. Duke's Phytochemical and Ethnobotanical Databases, Octane,[1]
