Medical gownsarehospitalgowns worn by medical professionals aspersonal protective equipment(PPE) in order to provide a barrier between patient and professional. Whereaspatient gownsare flimsy often with exposed backs and arms, PPE gowns, as seen below in the cardiac surgeon photograph, cover most of the exposed skin surfaces of the professional medics.

In several countries, PPE gowns for use in theCOVID-19 pandemicbecame in appearance more likecleanroom suitsas knowledge of thebest practicesfiltered up through the national bureaucracies. For example, the European norm-setting bodiesCENandCENELECon 30 March 2020 in collaboration with theEuropean Commissioner for the Internal Marketmade freely-available the relevant standards documents in order "to tackle the severe shortage of protective masks, gloves and other products currently faced by many European countries. Providing free access to the standards will facilitate the work of the many companies wishing to reconvert their production lines in order to manufacture the equipment that is so urgently needed."[2]
History
editThe concept of PPE in regards to medical professionals was seen as early as the 17th centuryPlague doctor's outfit.
During theEbola crisisof 2014, theWHOpublished a rapid advice guideline on PPE coveralls.[3]
Types
editThe different levels of various gown types are categorized as follows:[4]
| Level | Risk | Exposure | Product usable as/at | Protection levels | Tests |
|---|---|---|---|---|---|
| One | Minimum | Standard isolation, Basic care | Visitor gown | Allows small amount of fluid penetration. Slight barrier to fluids. | Only one test of water impacting the gown material's surface is conducted to determine barrier protection. |
| Two | Low | Surgical suturing,and during blood draw | Pathology lab,Intensive care unit | Protection from fluids for longer period than level one gowns. | Two tests
|
| Three | Moderate | Intravenous therapy,and to drawarterial blood | InTraumacases, or atEmergency | Protection from fluids for longer period than level two gowns. | Two tests
|
| Four | High | Surgery,and wherepathogen transmissionsuspected | Operating theater | Protection against fluids and virus for one hour. | Three tests
|
Local variants
editUnited States
editIn the United States, medical gowns aremedical devicesregulated by theFood and Drug Administration.FDA divides medical gowns into three categories. A surgical gown is intended to be worn by health care personnel during surgical procedures. Surgical isolation gowns are used when there is a medium to high risk of contamination and a need for larger critical zones of protection. Non-surgical gowns are worn in low or minimal risk situations.[5]
Surgical and surgical isolation gowns are regulated by the FDA as aClass II medical devicethat require a510(k) premarket notification,but non-surgical gowns are Class I devices exempt from premarket review. Surgical gowns only require protection of the front of the body due to the controlled nature of surgical procedures, while surgical isolation gowns and non-surgical gowns require protection over nearly the entire gown.[5]
In 2004, the FDA recognizedANSI/AAMIPB70:2003 standard on protective apparel and drapes for use in health care facilities. Surgical gowns must also conform to theASTMF2407 standard for tear resistance, seam strength, lint generation, evaporative resistance, and water vapor transmission. Because surgical gowns are considered to be a surface-contacting device with intact skin, FDA recommends that cytotoxicity, sensitization, and irritation or intracutaneous reactivity is evaluated.[5]
China
editThe First Affiliated Hospital of theZhejiang University School of MedicineinHangzhou,Zhejiang Province,People's Republic of Chinadeveloped their own protocol and equipment during the early months of theCOVID-19 pandemic.A screenshot of the cover of theHandbook of COVID-19 Prevention and Treatmentshows a picture of two rows of medical personnel, each wearing PPE gowns and PPE masks and PPE hoods and PPE goggles.
During theCOVID-19 pandemicinWuhan,doctors were provided with full PPE gown suits as early as January 2020.
European Union
editDuring theCOVID-19 pandemic,the European Commissioner for the Internal Market on 30 March 2020 listed the applicable norms for to help manufacturers re-convert their production lines:[2]
- Protective masks
- EN 149
- 2009-08: Respiratory protective devices – Filtering half masks to protect against particles - Requirements, testing, marking
- EN 14683
- 2019-10: Medical face masks - Requirements and test methods
- Eye protection
- EN 166
- 2002-04: Personal eye-protection – Specifications
- Protective clothing
- EN 14126
- 2004-01: Protective clothing - Performance requirements and tests methods for protective clothing against infective agents
- EN 14605
- 2009-08: Protective clothing against liquid chemicals - performance requirements for clothing with liquid-tight (Type 3) or spray-tight (Type 4) connections, including items providing protection to parts of the body only (Types PB [3] and PB [4])
- EN ISO 13688
- 2013-12 Protective clothing - General requirements (ISO 13688:2013)
- EN 13795-1
- 2019-06: Surgical clothing and drapes - Requirements and test methods – Part 1: Surgical drapes and gowns
- EN 13795-2
- 2019-06: Surgical clothing and drapes - Requirements and test methods – Part 2: Clean air suits
- Gloves
- EN 455-1
- 2001-01 Medical gloves for single use – Part 1: Requirements and testing for freedom from holes
- EN 455-2
- 2015-07: Medical gloves for single use – Part 2: Requirements and testing for physical properties
- EN 455-3
- 2015-07: Medical gloves for single use – Part 3: Requirements and testing for biological evaluation
- EN 455-4
- 2009-10: Medical gloves for single use – Part 4: Requirements and testing for shelf life determination
- EN 420
- 2010-03: Protective gloves - General requirements and test methods
- EN ISO 374-1
- 2018-10 Protective gloves against dangerous chemicals and micro-organisms – Part 1: Terminology and performance requirements for chemical risks
- EN ISO 374-5
- 2017-03: Protective gloves against dangerous chemicals and micro-organisms – Part 5: Terminology and performance requirements for micro-organisms risks (ISO 374-5:2016)
Israel
editAs seen in the accompanying gallery figure, at least oneIsraelihospital had access to fullTyvekPPE gowns as early as 17 March 2020 during theCOVID-19 pandemic.
Italy
editIn an early April article, 20 doctors from the whole of Italy describe their experience with coronavirus patient care. Their conclusion reads:[6]
Instituting precise well-established plans to perform undeferrable surgical procedures and emergencies on COVID-19-positive patient is mandatory. Hospitals must prepare specific internal protocols and arrange adequate training of the involved personnel.
Their findings are set out in a table entitled "Necessary personal protection equipment":
- FFP2 facial maskor (in case of maneuvers at high risk of generating aerosolized particles:) FFP3 facial mask
- Disposable long sleeve waterproof coats, gowns, orTyveksuits
- Disposable double pair ofnitrile gloves
- Protective goggles or visors
- Disposable head caps
- Disposable long shoe covers
- Alcoholic hand hygiene solution
Criticisms
editIn a May 2017 research article, several French scientists complained that there was little harmonization across Europe for the names ofpathogens,and went on to describe the PPE norms and regulations in France forinfectious diseasesunderBSL-3.[7]
See also
edit- Plague doctor's outfit– Clothing worn by plague doctors that was intended to protect them from infection, historical equivalent
- Hazmat suit
- Workplace hazard controls for COVID-19
References
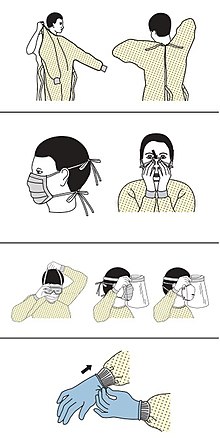
edit- ^"Sequence for Putting On Personal Protective Equipment (PPE)"(PDF).CDC.Archived(PDF)from the original on 5 March 2020.Retrieved8 March2020.
- ^ab"COVID-19: DIN makes standards for medical equipment available".DIN Deutsches Institut für Normung e. V. 2020-03-30.
- ^"Personal protective equipment for Ebola outbreak"(PDF).WHO. 31 October 2014.
- ^Health, Center for Devices and Radiological (2021-01-13)."Medical Gowns".FDA.
- ^abc"Medical Gowns".U.S. Food and Drug Administration.2020-03-11.Retrieved2020-05-06.
- ^Coccolini, F.; Perrone, G.; Chiarugi, M.; Di Marzo, F.; Ansaloni, L.; Scandroglio, I.; Marini, P.; Zago, M.; De Paolis, P.; Forfori, F.; Agresta, F.; Puzziello, A.; d'Ugo, D.; Bignami, E.; Bellini, V.; Vitali, P.; Petrini, F.; Pifferi, B.; Corradi, F.; Tarasconi, A.; Pattonieri, V.; Bonati, E.; Tritapepe, L.; Agnoletti, V.; Corbella, D.; Sartelli, M.; Catena, F. (2020)."Surgery in COVID-19 patients: Operational directives".World Journal of Emergency Surgery.15(1): 25.doi:10.1186/s13017-020-00307-2.PMC7137852.PMID32264898.
- ^Pastorino, Boris; De Lamballerie, Xavier; Charrel, Rémi (2017)."Biosafety and Biosecurity in European Containment Level 3 Laboratories: Focus on French Recent Progress and Essential Requirements".Frontiers in Public Health.5:121.doi:10.3389/fpubh.2017.00121.PMC5449436.PMID28620600.