This articleneeds additional citations forverification.(November 2022) |
Passive transportis a type ofmembrane transportthat does not requireenergyto move substances acrosscell membranes.[1][2]Instead of usingcellular energy,likeactive transport,[3]passive transport relies on thesecond law of thermodynamicsto drive the movement of substances across cell membranes.[1][2][4]Fundamentally, substances followFick's first law,and move from an area of high concentration to an area of low concentration because this movement increases theentropyof the overallsystem.[4][5]The rate of passive transport depends on thepermeabilityof the cell membrane, which, in turn, depends on the organization and characteristics of the membranelipidsandproteins.[citation needed]The four main kinds of passive transport are simplediffusion,facilitated diffusion,filtration,and/orosmosis.
Passive transport followsFick's first law.
Diffusion
editDiffusion is the net movement of material from an area of high concentration to an area with lower concentration. The difference of concentration between the two areas is often termed as theconcentration gradient,and diffusion will continue until this gradient has been eliminated. Since diffusion moves materials from an area of higher concentration to an area of lower concentration, it is described as moving solutes "down the concentration gradient" (compared withactive transport,which often moves material from area of low concentration to area of higher concentration, and therefore referred to as moving the material "against the concentration gradient" ). However, in many cases (e.g. passive drug transport) the driving force of passive transport can not be simplified to the concentration gradient. If there are different solutions at the two sides of the membrane with different equilibrium solubility of the drug, the difference in the degree of saturation is the driving force of passive membrane transport.[6]It is also true for supersaturated solutions which are more and more important owing to the spreading of the application of amorphous solid dispersions for drugbioavailabilityenhancement.
Simple diffusion and osmosis are in some ways similar. Simple diffusion is the passive movement of solute from a high concentration to a lower concentration until the concentration of the solute is uniform throughout and reaches equilibrium. Osmosis is much like simple diffusion but it specifically describes the movement of water (not the solute) across a selectively permeable membrane until there is an equal concentration of water and solute on both sides of the membrane. Simple diffusion and osmosis are both forms of passive transport and require none of the cell'sATP energy.
Speed of diffusion
editFor passive diffusion, the law of diffusion states that the mean squared displacement iswithdbeing the number of dimensions andDthediffusion coefficient). So to diffuse a distance of abouttakes time,and the "average speed" is.This means that in the same physical environment, diffusion is fast when the distance is small, but less when the distance is large.
This can be seen in material transport within the cell. Prokaryotes typically have small bodies, allowing diffusion to suffice for material transport within the cell. Larger cells like eukaryotes would either have very low metabolic rate to accommodate the slowness of diffusion, or invest in complex cellular machinery to allow active transport within the cell, such askinesinwalking alongmicrotubules.
Example of diffusion: gas exchange
editA biological example of diffusion is thegas exchangethat occurs duringrespirationwithin the human body.[7]Upon inhalation,oxygenis brought into thelungsand quickly diffuses across the membrane ofalveoliand enters thecirculatory systemby diffusing across the membrane of the pulmonarycapillaries.[8]Simultaneously,carbon dioxidemoves in the opposite direction, diffusing across the membrane of the capillaries and entering into the alveoli, where it can be exhaled. The process of moving oxygen into the cells, and carbon dioxide out, occurs because of the concentration gradient of these substances, each moving away from their respective areas of higher concentration toward areas of lower concentration.[7][8]Cellular respirationis the cause of the low concentration of oxygen and high concentration of carbon dioxide within the blood which creates the concentration gradient. Because the gasses are small and uncharged, they are able to pass directly through thecell membranewithout any special membrane proteins.[9]No energy is required because the movement of the gasses followsFick's first lawand thesecond law of thermodynamics.
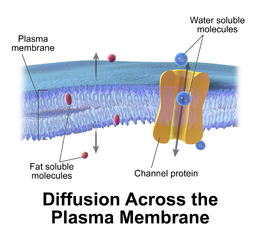
Facilitated diffusion
editFacilitated diffusion, also called carrier-mediated osmosis, is the movement of molecules across the cell membrane via special transport proteins that are embedded in the plasma membrane by actively taking up or excluding ions[14].Through facilitated diffusion, energy is not required in order for molecules to pass through the cell membrane.[1]Active transport ofprotonsbyH+ATPases[10]altersmembrane potentialallowing for facilitated passive transport of particular ions such as potassium[11]down their charge gradient through high affinity transporters and channels.
Example of facilitated diffusion: GLUT2
editAn example offacilitated diffusionis whenglucoseis absorbed into cells throughGlucose transporter 2 (GLUT2)in the human body.[12][13]There are many other types ofglucose transport proteins,some that dorequire energy,and are therefore not examples of passive transport.[13]Since glucose is a large molecule, it requires aspecific channelto facilitate its entry acrossplasma membranesand into cells.[13]When diffusing into a cell through GLUT2, the driving force that moves glucose into the cell is the concentration gradient.[12]The main difference betweensimple diffusionandfacilitated diffusionis that facilitated diffusion requires atransport proteinto 'facilitate' or assist the substance through the membrane.[14]After a meal, the cell is signaled to move GLUT2 into membranes of the cells lining the intestines calledenterocytes.[12]With GLUT2 in place after a meal and the relative high concentration of glucose outside of these cells as compared to within them, the concentration gradient drives glucose across the cell membrane through GLUT2.[12][13]
Filtration
editFiltration is movement of water and solute molecules across the cell membrane due to hydrostaticpressuregenerated by thecardiovascular system.Depending on the size of the membrane pores, only solutes of a certain size may pass through it. For example, the membrane pores of theBowman's capsulein the kidneys are very small, and onlyalbumins,the smallest of the proteins, have any chance of being filtered through. On the other hand, the membrane pores oflivercells are extremely large, but not forgetting cells are extremely small to allow a variety of solutes to pass through and be metabolized.
Osmosis
editOsmosis is the net movement of watermoleculesacross a selectively permeable membrane from an area of high water potential to an area of low water potential. A cell with a less negative water potential will draw in water, but this depends on other factors as well such as solute potential (pressure in the cell e.g. solute molecules) and pressure potential (external pressure e.g. cell wall). There are three types of Osmosis solutions: the isotonic solution, hypotonic solution, and hypertonic solution. Isotonic solution is when the extracellular solute concentration is balanced with the concentration inside the cell. In the Isotonic solution, the water molecules still move between the solutions, but the rates are the same from both directions, thus the water movement is balanced between the inside of the cell as well as the outside of the cell. A hypotonic solution is when the solute concentration outside the cell is lower than the concentration inside the cell. In hypotonic solutions, the watermoves intothe cell, down its concentration gradient (from higher to lower water concentrations). That can cause the cell to swell. Cells that don't have a cell wall, such as animal cells, could burst in this solution. A hypertonic solution is when the solute concentration is higher (think of hyper - as high) than the concentration inside the cell. In hypertonic solution, the water willmove out,causing the cell to shrink.
See also
editReferences
edit- ^abc"5.2 Passive Transport - Biology 2e | OpenStax".openstax.org.28 March 2018.Retrieved2020-12-06.
- ^ab"5.2A: The Role of Passive Transport".Biology LibreTexts.2018-07-10.Retrieved2020-12-06.
- ^"5.3 Active Transport - Biology 2e | OpenStax".openstax.org.28 March 2018.Retrieved2020-12-06.
- ^abSkene, Keith R. (2015)."Life's a Gas: A Thermodynamic Theory of Biological Evolution".Entropy.17(8): 5522–5548.Bibcode:2015Entrp..17.5522S.doi:10.3390/e17085522.
- ^"12.7 Molecular Transport Phenomena: Diffusion, Osmosis, and Related Processes - College Physics for AP® Courses | OpenStax".openstax.org.12 August 2015.Retrieved2020-12-06.
- ^Borbas, E.; et al. (2016). "Investigation and Mathematical Description of the Real Driving Force of Passive Transport of Drug Molecules from Supersaturated Solutions".Molecular Pharmaceutics.13(11): 3816–3826.doi:10.1021/acs.molpharmaceut.6b00613.PMID27611057.
- ^abWagner, Peter D. (2015-01-01)."The physiological basis of pulmonary gas exchange: implications for clinical interpretation of arterial blood gases".European Respiratory Journal.45(1): 227–243.doi:10.1183/09031936.00039214.ISSN0903-1936.PMID25323225.
- ^ab"22.4 Gas Exchange - Anatomy and Physiology | OpenStax".openstax.org.25 April 2013.Retrieved2020-12-06.
- ^"3.1 The Cell Membrane - Anatomy and Physiology | OpenStax".openstax.org.25 April 2013.Retrieved2020-12-06.
- ^Palmgren, Michael G. (2001-01-01). "PLANT PLASMA MEMBRANE H+-ATPases: Powerhouses for Nutrient Uptake".Annual Review of Plant Physiology and Plant Molecular Biology.52(1): 817–845.doi:10.1146/annurev.arplant.52.1.817.PMID11337417.
- ^Dreyer, Ingo; Uozumi, Nobuyuki (2011-11-01)."Potassium channels in plant cells".FEBS Journal.278(22): 4293–4303.doi:10.1111/j.1742-4658.2011.08371.x.ISSN1742-4658.PMID21955642.S2CID12814450.
- ^abcdKellett, George L.; Brot-Laroche, Edith; Mace, Oliver J.; Leturque, Armelle (2008)."Sugar absorption in the intestine: the role of GLUT2".Annual Review of Nutrition.28:35–54.doi:10.1146/annurev.nutr.28.061807.155518.ISSN0199-9885.PMID18393659.
- ^abcdChen, Lihong; Tuo, Biguang; Dong, Hui (2016-01-14)."Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters".Nutrients.8(1): 43.doi:10.3390/nu8010043.ISSN2072-6643.PMC4728656.PMID26784222.
- ^Cooper, Geoffrey M. (2000)."Transport of Small Molecules".The Cell: A Molecular Approach. 2nd Edition.
- Alcamo, I. Edward (1997). "Chapter 2–5: Passive transport".Biology coloring workbook.Illustrations by John Bergdahl. New York: Random House. pp. 24–25.ISBN9780679778844.
- Sadava, David; H. Craig Heller; Gordon H. Orians; William K. Purves; David M. Hillis (2007)."What are the passive processes of membrane transport?".Life: the science of biology(8th ed.). Sunderland, MA: Sinauer Associates. pp.105–110.ISBN9780716776710.
- Srivastava, P. K. (2005).Elementary biophysics: an introduction.Harrow: Alpha Science Internat. pp. 140–148.ISBN9781842651933.