Picornavirusesare a group of relatednonenvelopedRNA viruseswhich infectvertebratesincludingfish,[2]mammals,andbirds.They arevirusesthat represent a large family of small,positive-sense, single-stranded RNA viruseswith a 30 nmicosahedralcapsid.The viruses in this family can cause a range of diseases including thecommon cold,poliomyelitis,meningitis,hepatitis,andparalysis.[3][4][5][6]
| Picornaviridae | |
|---|---|
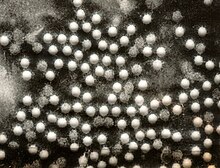
| |
| Electronmicrographofpoliovirus | |
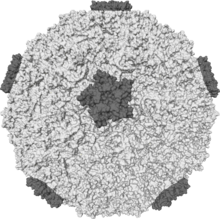
| |
| Isosurface of a humanrhinovirusshowing protein spikes | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Pisuviricota |
| Class: | Pisoniviricetes |
| Order: | Picornavirales |
| Family: | Picornaviridae |
| Genera[1] | |
Picornaviruses constitute thefamilyPicornaviridae,orderPicornavirales,and realmRiboviria.There are 158 species in this family, assigned to 68 genera. Notable examples are generaEnterovirus(includingRhinovirusandPoliovirus),Aphthovirus,Cardiovirus,andHepatovirus.[1][7]
Etymology
editThe name "picornavirus" has a dualetymology.Firstly, the name derives frompicorna- which is anacronymfor "poliovirus,insensitivity toether,coxsackievirus,orphan virus,rhinovirus, andribonucleicacid".Secondly, the name derives frompico-,which designates a very small unit of measurement (equivalent to 10−12), combined withrnato describe this group of very smallRNA viruses.[8]
History
editThe first animal virus discovered (1897) was thefoot-and-mouth diseasevirus (FMDV). It is the prototypic member of the genusAphthovirusin thePicornaviridaefamily.[5]Theplaqueassay was developed usingpoliovirus;the discovery ofviral replicationin culture was also with poliovirus in 1949. This was the first time that infectious virus had been produced in cultured cells.[9]Polyprotein synthesis,internal ribosome entry sites,anduncappedmRNAwere all discovered by studying poliovirus infected cells, and a poliovirus clone was the first infectiousDNA clonemade of anRNA virusin animals. Along withrhinovirus,poliovirus was the first animal virus to have its structure determined byx-ray crystallography.RNA dependent RNA polymerasewas discovered inMengovirus,a genus of picornaviruses.[10]
Virology
editStructure
editPicornaviruses are nonenveloped, with anicosahedralcapsid.[4]The capsid is an arrangement of 60protomersin a tightly packed icosahedral structure. Each protomer consists of fourpolypeptidesknown as VP (viral protein) 1, 2, 3 and 4. VP2 and VP4 polypeptides originate from one protomer known as VP0 that is cleaved to give the different capsid components. The icosahedral capsid is said to have atriangulation numberof 3, this means that in the icosahedral structure each of the 60 triangles that make up the capsid are split into three little triangles with a subunit on the corner.[citation needed]
Many picornaviruses have a deep cleft formed by around each of the 12 vertices of icosahedrons. The outer surface of the capsid is composed of regions of VP1, VP2, and VP3. Around each of the vertices is a canyon lined with the C termini of VP1 and VP3. The interior surface of the capsid is composed of VP4 and the N termini of VP1. J. Esposito andFrederick A. Murphydemonstrates cleft structure referred to as canyons, using X-ray crystallography and cryoelectron microscopy.[9]
Depending on the type and degree of dehydration, the viral particle is around 30–32 nm in diameter.[7]The viral genome is around 2500 nm in length, so it is tightly packaged within the capsid along with substances such assodiumions to balance the negative charges on the RNA caused by thephosphategroups.[citation needed]
Genome
editPicornaviruses are classed underBaltimore's viral classification systemas group IV viruses, as they contain a single-stranded, positive-sense RNAgenome.Their genome ranges between 6.7 and 10.1 (kilobases) in length.[7]Like most positive-sense RNA genomes, the genetic material alone is infectious; although substantially lessvirulentthan if contained within the viral particle, the RNA can have increased infectivity when transfected into cells. The genomeRNAis unusual because it has aproteinon the 5' end that is used as aprimerfortranscriptionbyRNA polymerase.This primer is called VPg genome, and it ranges between 2 and 3 kb. VPg contain tyrosine residue at the 3' end. Tyrosine as a –OH source for covalently linked to 5' end of RNA.[9][11]
The genome is not segmented and positive-sense (the same sense as mammalian mRNA, being read 5' to 3'). UnlikemammalianmRNA,picornaviruses do not have a5' cap,but a virally encoded protein known asVPg.However, like mammalian mRNA, the genome does have apoly(A) tailat the 3' end. An untranslated region (UTR) is found at both ends of the picornavirus genome. The 5' UTR is usually longer, being around 500–1200 nucleotides (nt) in length, compared to that of the 3' UTR, which is around 30–650 nt. The 5' UTR is thought to be important in translation, and the 3' in negative-strand synthesis; however, the 5' end may also have a role to play in virulence of the virus. The rest of the genome encodes structural proteins at the 5' end and nonstructural proteins at the 3' end in a single polyprotein.[citation needed]
The polyprotein is organised as: L-1ABCD-2ABC-3ABCD with each letter representing a protein, but variations to this layout exist.[citation needed]
The 1A, 1B, 1C, and 1D proteins are the capsid proteins VP4, VP2, VP3, and VP1, respectively. Virus-coded proteases perform the cleavages, some of which are intramolecular. The polyprotein is first cut to yield P1, P2, and P3. P1 becomes myristylated at the N terminus before being cleaved to VP0, VP3, and VP1, the proteins that will form procapsids; VP0 will later be cleaved to produce VP2 and VP4. Other cleavage products include 3B (VPg), 2C (an ATPase), and 3D (the RNA polymerase).[9][12]
Replication
editRNA elements
editGenomic RNAs of picornaviruses possess multiple RNA elements, and they are required for both negative- and positive-strand RNA synthesis. The cis-acting replication element (CRE) is required for replication. The stem-loop-structure that contains the CRE is independent of position, but changes with location between virus types when it has been identified. Also, the 3' end elements of viral RNA are significant and efficient for RNA replication of picornaviruses. The 3' end of picornavirus contains a poly(A) tract, which is required for infectivity. RNA synthesis, though, is hypothesized to occur in this region. The 3' end NCR of poliovirus is not necessary for negative-strand synthesis, but is important element for positive–strand synthesis. Additionally, the 5' end NCR that contains secondary structural elements is required for RNA replication and poliovirus translation initiation. Internal ribosome entry sites are RNA structures that allow cap-independent initiation of translation, and are able to initiate translation in the middle of a messenger RNA.[13]
Lifecycle
editThe viral particle binds to cell surface receptors. Cell surface receptors are characterized for each serotype of picornaviruses. For example, poliovirus receptor is glycoprotein CD155, which is special receptor for human and some other primate species. For this reason, poliovirus could not be made in many laboratories until transgenic mice having a CD155 receptor on their cell surfaces were developed in the 1990s. These animals can be infected and used for studies of replication and pathogenesis.[9]Binding causes a conformational change in the viral capsid proteins, andmyristic acidis released. The acid forms a pore in the cell membrane through which RNA is injected.[14]
Once inside the cell, the RNA uncoats and the (+) strand RNA genome is replicated through a double-stranded RNA intermediate that is formed using viral RNA-dependent RNA polymerase. Translation by host-cell ribosomes is not initiated by a 5' G cap as usual, but rather is initiated by an internal ribosome entry site. The viral life cycle is very rapid, with the whole process of replication being completed on average within 8 hours. As little as 30 minutes after initial infection, though, cell protein synthesis declines to almost zero output – essentially the macromolecular synthesis of cell proteins is shut off. Over the next 1–2 hours, a loss of margination ofchromatinandhomogeneityoccurs in the nucleus, before the viral proteins start to be synthesized and a vacuole appears in the cytoplasm close to the nucleus that gradually starts to spread as the time after infection reaches around 3 hours. After this time, the cell plasma membrane becomes permeable; at 4–6 hours, the virus particles assemble, and can sometimes be seen in the cytoplasm. Around 8 hours, the cell is effectively dead, and lyses to release the viral particles.[citation needed]
Experimental data from single-step growth curve-like experiments have allowed observation of the replication of the picornaviruses in great detail. The whole of replication occurs within the host-cell cytoplasm and infection can even happen in cells that do not contain anucleus(enucleated) and those treated withactinomycin D(this antibiotic would inhibit viral replication if this occurred in the nucleus.)[citation needed]
Translation takes place by -1 ribosomal frameshifting, viral initiation, and ribosomal skipping. The virus exits in host cell by lysis, and viroporins. Vertebrates serve as the natural hosts. Transmission routes are fecal-oral, contact, ingestion, and air-borne particles.[4]
Viral protein (VPg)
editPicornaviruses have a viral protein (VPg) covalently linked to 5' end of their genomes instead of 7-methylguanosine cap like cellular mRNAs. Virus RNA polymerases use VPg as primer. VPg as primer uses both positive- and negative-strand RNA synthesis. Picornavirus replication is initiated by the uridylylation of VPg. It is uridylylated at the hydroxyl group of a tyrosine residue.[3]A VPg primer mechanism is used by the picornavirus (entero- aphtho-, and others), additional virus groups (poty-, como-, calici-, and others) and picornavirus-like (coronavirus, notavirus, etc.) supergroup of RNA viruses. The mechanism has been best studied for the enteroviruses (which include many human pathogens, such aspoliovirusandcoxsackie viruses), as well as for the aphthovirus, an animal pathogen causing foot-and-mouth disease.[citation needed]
In this group, primer-dependent RNA synthesis uses a small 22– to 25-amino acid-long viral protein linked to the VPg[15]to initiate polymerase activity, where the primer is covalently bound to the 5' end of the RNA template.[16]The uridylylation occurs at a tyrosine residue at the third position of the VPg. A CRE, which is a RNA stem loop structure, serves as a template for the uridylylation of VPg, resulting in the synthesis of VPgpUpUOH. Mutations within the CRE-RNA structure prevent VPg uridylylation, and mutations within the VPg sequence can severely diminish RdRp catalytic activity.[17]While the tyrosine hydroxyl of VPg can prime negative-strand RNA synthesis in a CRE- and VPgpUpUOH-independent manner, CRE-dependent VPgpUpUOH synthesis is absolutely required for positive-strand RNA synthesis. CRE-dependent VPg uridylylation lowers the Km¬of UTP required for viral RNA replication and CRE-dependent VPgpUpUOH synthesis, and is required for efficient negative-strand RNA synthesis, especially when UTP concentrations are limiting.[18]The VPgpUpUOH primer is transferred to the 3’ end of the RNA template for elongation, which can continue by addition of nucleotide bases by RdRp. Partial crystal structures for VPgs of foot and mouth disease virus[19]and coxsackie virus B3[20]suggest that there may be two sites on the viral polymerase for the small VPgs of the picornaviruses. NMR solution structures of poliovirus VPg[21]and VPgpU[22]show that uridylylation stabilizes the structure of the VPg, which is otherwise quite flexible in solution. The second site may be used for uridylylation,v after which the VPgpU can initiate RNA synthesis. The VPg primers of caliciviruses, whose structures are only beginning to be revealed,[23]are much larger than those of the picornaviruses. Mechanisms for uridylylation and priming may be quite different in all of these groups.[citation needed]
VPg uridylylation may include the use of precursor proteins, allowing for the determination of a possible mechanism for the location of the diuridylylated, VPg-containing precursor at the 3' end of positive- or negative-strand RNA for production of full-length RNA. Determinants of VPg uridylylation efficiency suggest formation and/or collapse or release of the uridylylated product as the rate-limiting stepin vitrodepending upon the VPg donor employed.[24]Precursor proteins also have an effect on VPg-CRE specificity and stability.[25]The upper RNA stem loop, to which VPg binds, has a significant impact on both retention, and recruitment, of VPg and Pol. The stem loop of CRE will partially unwind, allowing the precursor components to bind and recruit VPg and Pol4. The CRE loop has a defined consensus sequence to which the initiation components bind, but no consensus sequence exists for the supporting stem, which suggests that only the structural stability of the CRE is important.[26]
Assembly and organization of the picornavirus VPg ribonucleoprotein complex
edit- Two 3CD (VPg complex) molecules bind to CRE with the 3C domains (VPg domain) contacting the upper stem and the 3D domains (VPg domain) contacting the lower stem.
- The 3C dimer opens the RNA stem by forming a more stable interaction with single strands forming the stem.
- 3Dpol is recruited to and retained in this complex by a physical interaction between the back-of-the-thumb subdomain of 3Dpol and a surface of one or both 3C subdomains of 3CD.
VPg may also play an important role in specific recognition of viral genome by movement proteins (MP), which are nonstructural proteins encoded by many, if not all, plant viruses to enable their movement from one infected cell to neighboring cells.[27]MP and VPg interact to provide specificity for the transport of viral RNA from cell to cell. To fulfill energy requirements, MP also interacts with P10, which is a cellular ATPase.[citation needed]
Diseases
editPicornaviruses cause a range of diseases. Enteroviruses of the picornavirus family infect theenterictract, which is reflected in their name. Rhinoviruses infect primarily thenoseand thethroat.Enteroviruses replicate at 37 °C, whereasrhinovirusesgrow better at 33 °C, as this is the lower temperature of the nose. Enteroviruses are stable under acidic conditions, thus they are able to survive exposure togastric acid.In contrast, rhinoviruses are acid-labile (inactivated or destroyed by lowpHconditions), so rhinovirus infections are restricted to the nose and throat.[citation needed]
Taxonomy
editThese genera are recognized:[1]
- Aalivirus
- Ailurivirus
- Ampivirus
- Anativirus
- Aphthovirus
- Aquamavirus
- Avihepatovirus
- Avisivirus
- Boosepivirus
- Bopivirus
- Caecilivirus
- Cardiovirus
- Cosavirus
- Crahelivirus
- Crohivirus
- Danipivirus
- Dicipivirus
- Diresapivirus
- Enterovirus
- Erbovirus
- Felipivirus
- Fipivirus
- Gallivirus
- Gruhelivirus
- Grusopivirus
- Harkavirus
- Hemipivirus
- Hepatovirus
- Hunnivirus
- Kobuvirus
- Kunsagivirus
- Limnipivirus
- Livupivirus
- Ludopivirus
- Malagasivirus
- Marsupivirus
- Megrivirus
- Mischivirus
- Mosavirus
- Mupivirus
- Myrropivirus
- Orivirus
- Oscivirus
- Parabovirus
- Parechovirus
- Pasivirus
- Passerivirus
- Pemapivirus
- Poecivirus
- Potamipivirus
- Pygoscepivirus
- Rabovirus
- Rafivirus
- Rajidapivirus
- Rohelivirus
- Rosavirus
- Sakobuvirus
- Salivirus
- Sapelovirus
- Senecavirus
- Shanbavirus
- Sicinivirus
- Symapivirus
- Teschovirus
- Torchivirus
- Tottorivirus
- Tremovirus
- Tropivirus
See also
editReferences
edit- ^abc"Virus Taxonomy: 2020 Release".International Committee on Taxonomy of Viruses (ICTV). March 2021.Retrieved20 May2021.
- ^Altan E, Kubiski SV, Boros Á, Reuter G, Sadeghi M, Deng X, Creighton EK, Crim MJ, Delwart E (2019)."A highly divergent picornavirus infecting the Gut Epithelia of Zebrafish (Danio rerio) in research institutions worldwide".Zebrafish.16(3): 291–299.doi:10.1089/zeb.2018.1710.PMID30939077.S2CID92999901.
- ^abRyu WS (2016). "Chapter 11 – Picornavirus".Molecular virology of human pathogenic viruses.Korea: Academic Press. pp. 153–64.doi:10.1016/b978-0-12-800838-6.00011-4.ISBN978-0-12-800838-6.
- ^abc"Viral Zone".ExPASy.Retrieved15 June2015.
- ^abMartinez-Salas E, Saiz M, Sobrino F (2008). "Foot-and-Mouth Disease Virus". In Mettenleiter TC, Sobrino F (eds.).Animal Viruses: Molecular Biology.Norfolk, UK: Caister Academic Press.ISBN978-1-904455-22-6.
- ^Lau SK, Woo PC, Lai KK, Huang Y, Yip CC, Shek CT, Lee P, Lam CS, Chan KH, Yuen KY (September 2011)."Complete genome analysis of three novel picornaviruses from diverse bat species".Journal of Virology.85(17): 8819–28.doi:10.1128/JVI.02364-10.PMC3165794.PMID21697464.
- ^abc"Picornaviridae - Picornaviridae - Picornavirales".International Committee on Taxonomy of Viruses (ICTV).Archived fromthe originalon 21 September 2017.Retrieved12 June2020.
- ^"Picornaviridae".International Committee on Taxonomy of Viruses (ICTV).October 2017. Archived fromthe originalon 21 September 2017.Retrieved5 February2019.
- ^abcdeCarter JB, Saunders VA (2007). "Picornaviruses (and other plus-strand RNA viruses)".Virology: Principles and applications.Chichester, England: John Wiley & Sons. pp. 160–65.ISBN978-0-470-02386-0.
- ^Knipe DM, Howley P (2013).Fields Virology.Lippincott Williams & Wilkins.ISBN978-1-4698-3066-7.
- ^Zabel P, Moerman M, Lomonossoff G, Shanks M, Beyreuther K (July 1984)."Cowpea mosaic virus VPg: sequencing of radiochemically modified protein allows mapping of the gene on B RNA".The EMBO Journal.3(7): 1629–34.doi:10.1002/j.1460-2075.1984.tb02021.x.PMC557569.PMID16453534.
- ^Acheson NH (2011).Fundamentals of Molecular Virology(2nd ed.). John Wiley & Sons, Inc.ISBN978-0470900598.
- ^Daijogo S, Semler BL (2011). "Mechanistic intersections between picornavirus translation and RNA replication".Advances in Virus Research.80:1–24.doi:10.1016/B978-0-12-385987-7.00001-4.ISBN9780123859877.PMID21762819.
- ^"Pathology, Microbiology and Immunology - School of Medicine Columbia | University of South Carolina".
- ^Flanegan JB, Baltimore D (September 1977)."Poliovirus-specific primer-dependent RNA polymerase able to copy poly(A)".Proceedings of the National Academy of Sciences of the United States of America.74(9): 3677–80.Bibcode:1977PNAS...74.3677F.doi:10.1073/pnas.74.9.3677.PMC431685.PMID198796.
- ^Ambros V, Baltimore D (August 1978)."Protein is linked to the 5' end of poliovirus RNA by a phosphodiester linkage to tyrosine".The Journal of Biological Chemistry.253(15): 5263–66.doi:10.1016/S0021-9258(17)30361-7.PMID209034.
- ^Gu C, Zeng T, Li Y, Xu Z, Mo Z, Zheng C (October 2009). "Structure-function analysis of mutant RNA-dependent RNA polymerase complexes with VPg".Biochemistry. Biokhimiia.74(10): 1132–41.doi:10.1134/S0006297909100095.PMID19916926.S2CID24968119.
- ^Steil BP, Barton DJ (October 2008)."Poliovirus cis-acting replication element-dependent VPg Uridylylation lowers the Km of the initiating nucleoside triphosphate for viral RNA replication".Journal of Virology.82(19): 9400–08.doi:10.1128/JVI.00427-08.PMC2546976.PMID18653453.
- ^Ferrer-Orta C, Arias A, Agudo R, Pérez-Luque R, Escarmís C, Domingo E, Verdaguer N (February 2006)."The structure of a protein primer-polymerase complex in the initiation of genome replication".The EMBO Journal.25(4): 880–88.doi:10.1038/sj.emboj.7600971.PMC1383552.PMID16456546.
- ^Gruez A, Selisko B, Roberts M, Bricogne G, Bussetta C, Jabafi I, et al. (October 2008)."The crystal structure of coxsackievirus B3 RNA-dependent RNA polymerase in complex with its protein primer VPg confirms the existence of a second VPg binding site on Picornaviridae polymerases".Journal of Virology.82(19): 9577–90.doi:10.1128/JVI.00631-08.PMC2546979.PMID18632861.
- ^Schein CH, Oezguen N, Volk DE, Garimella R, Paul A, Braun W (July 2006)."NMR structure of the viral peptide linked to the genome (VPg) of poliovirus".Peptides.27(7): 1676–84.doi:10.1016/j.peptides.2006.01.018.PMC1629084.PMID16540201.
- ^Schein CH, Oezguen N, van der Heden van Noort GJ, Filippov DV, Paul A, Kumar E, Braun W (August 2010)."NMR solution structure of poliovirus uridylyated peptide linked to the genome (VPgpU)".Peptides.31(8): 1441–48.doi:10.1016/j.peptides.2010.04.021.PMC2905501.PMID20441784.
- ^Leen EN, Kwok KY, Birtley JR, Simpson PJ, Subba-Reddy CV, Chaudhry Y, et al. (May 2013)."Structures of the compact helical core domains of feline calicivirus and murine norovirus VPg proteins"(PDF).Journal of Virology.87(10): 5318–30.doi:10.1128/JVI.03151-12.PMC3648151.PMID23487472.
- ^Pathak HB, Oh HS, Goodfellow IG, Arnold JJ, Cameron CE (November 2008)."Picornavirus genome replication: roles of precursor proteins and rate-limiting steps in oriI-dependent VPg uridylylation".The Journal of Biological Chemistry.283(45): 30677–88.doi:10.1074/jbc.M806101200.PMC2576561.PMID18779320.
- ^Shen M, Wang Q, Yang Y, Pathak HB, Arnold JJ, Castro C, Lemon SM, Cameron CE (November 2007)."Human Rhinovirus Type 14 Gain-of-Function Mutants for oriI Utilization Define Residues of 3C(D) and 3Dpol That Contribute to Assembly and Stability of the Picornavirus VPg Uridylylation Complex".J. Virol.81(22): 12485–95.doi:10.1128/JVI.00972-07.PMC2169002.PMID17855535.
- ^Yang Y, Rijnbrand R, McKnight KL, Wimmer E, Paul A, Martin A, Lemon SM (August 2002)."Sequence requirements for viral RNA replication and VPg uridylylation directed by the internal cis-acting replication element (CRE) of human rhinovirus type 14".Journal of Virology.76(15): 7485–94.doi:10.1128/JVI.76.15.7485-7494.2002.PMC136355.PMID12097561.
- ^Roy Chowdhury S, Savithri HS (January 2011). Pfeffer S (ed.)."Interaction of Sesbania mosaic virus movement protein with VPg and P10: implication to specificity of genome recognition".PLOS ONE.6(1): e15609.Bibcode:2011PLoSO...615609R.doi:10.1371/journal.pone.0015609.PMC3016346.PMID21246040.
Further reading
edit- Kahn CM, Line S, eds. (2005)."Porcine Enteroviral Encephalomyelitis".The Merck Veterinary Manual(9th ed.).Merck.ISBN978-0-911910-50-6.
- Thompson JR, Dasgupta I, Fuchs M, Iwanami T, Karasev AV, Petrzik K, Sanfaçon H, Tzanetakis I, van der Vlugt R, Wetzel T, Yoshikawa N, et al. (ICTV Report Consortium) (April 2017)."ICTV Virus Taxonomy Profile: Secoviridae".The Journal of General Virology.98(4): 529–531.doi:10.1099/jgv.0.000779.PMC5657025.PMID28452295.
- Thompson JR (2020). "Secoviruses (Secoviridae)".Reference Module in Life Sciences.pp. 692–702.doi:10.1016/B978-0-12-809633-8.21253-3.ISBN978-0-12-809633-8.S2CID241194454.
External links
edit- Büchen-Osmond C, ed. (2006)."ICTVdB – The Universal Virus Database, version 4".ICTVdB Management, Mailman School of Public Health.New York: Columbia University. Archived fromthe originalon 10 March 2010.
- "Picornaviridae".Medical Subject Headings(MeSH).National Library of Medicine.Retrieved3 September2007.
- "Picornavirus".The Pirbright Institute.Retrieved3 September2007.
- ICTV Online ReportPicornaviridae
- ICTV International Committee on Taxonomy of Viruses Master Species ListsArchived5 March 2016 at theWayback Machine
- ICTV International Committee on Taxonomy of Viruses (official site)Archived3 February 2017 at theWayback Machine
- Picornaviruses– description, replication, disease
- Picornaviruses in NCBI Taxonomy browser
- Picornaviridaeclassificationby the International Committee on Taxonomy of Viruses
- Animal viruses
- Viralzone:Picornaviridae
- Virus Pathogen Database and Analysis Resource (ViPR): Picornaviridae
- ICTV