Thesomites(outdated term:primitive segments) are a set of bilaterally paired blocks ofparaxial mesodermthat form in theembryonic stageofsomitogenesis,along the head-to-tail axis insegmentedanimals. Invertebrates,somites subdivide into the dermatomes,myotomes, sclerotomesandsyndetomesthat give rise to thevertebraeof thevertebral column,rib cage,part of theoccipital bone,skeletal muscle,cartilage,tendons,andskin(of the back).[2]
| Somite | |
|---|---|
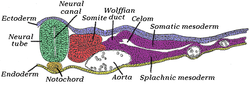 Transverse section of half of a chick embryo of forty-five hours' incubation. The dorsal (back) surface of the embryo is towards the top of this page, while the ventral (front) surface is towards the bottom. | |
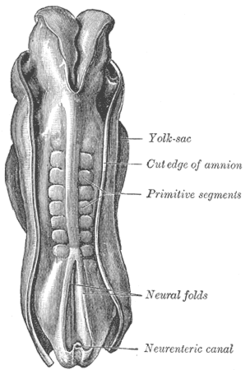 Dorsum of human embryo, 2.11 mm in length. (The older termprimitive segmentsis used to identify the somites.) | |
| Details | |
| Carnegie stage | 9 |
| Days | 20[1] |
| Precursor | Paraxial mesoderm |
| Gives rise to | Dermatome,myotome, sclerotome,syndetome |
| Identifiers | |
| Latin | somitus |
| MeSH | D019170 |
| TE | E5.0.2.2.2.0.3 |
| FMA | 85522 |
| Anatomical terminology | |
The wordsomiteis sometimes also used in place of the wordmetamere.In this definition, the somite is ahomologously-paired structure in an animalbody plan,such as is visible inannelidsandarthropods.[3]
Development
editThemesodermforms at the same time as the other twogerm layers,theectodermandendoderm.The mesoderm at either side of the neural tube is calledparaxial mesoderm.It is distinct from the mesoderm underneath the neural tube, which is called thechordamesodermthat becomes the notochord. The paraxial mesoderm is initially called the "segmental plate" in the chick embryo or the "unsegmented mesoderm" in other vertebrates. As theprimitive streakregresses and neural folds gather (to eventually become theneural tube), the paraxial mesoderm separates into blocks called somites.[4]
Formation
editThe pre-somitic mesoderm commits to the somitic fate before mesoderm becomes capable of forming somites. The cells within each somite are specified based on their location within the somite. Additionally, they retain the ability to become any kind of somite-derived structure until relatively late in the process ofsomitogenesis.[4]
The development of the somites depends on a clock mechanism as described by theclock and wavefront model.In one description of the model, oscillatingNotchandWntsignals provide the clock. The wave is a gradient of thefibroblast growth factorprotein that isrostral to caudal(nose to tail gradient). Somites form one after the other down the length of the embryo from the head to the tail, with each new somite forming on the caudal (tail) side of the previous one.[5][6]
The timing of the interval is not universal. Different species have different interval timing. In thechickembryo, somites are formed every 90 minutes. In themousethe interval is 2 hours.[7]
For some species, the number of somites may be used to determine the stage of embryonic development more reliably than the number of hours post-fertilization because rate of development can be affected by temperature or other environmental factors. The somites appear on both sides of the neural tube simultaneously. Experimental manipulation of the developing somites will not alter the rostral/caudal orientation of the somites, as the cell fates have been determined prior to somitogenesis. Somite formation can be induced byNoggin-secreting cells. The number of somites is species dependent and independent of embryo size (for example, if modified via surgery or genetic engineering). Chicken embryos have 50 somites; mice have 65, while snakes have 500.[4][8]
As cells within the paraxial mesoderm begin to come together, they are termedsomitomeres,indicating a lack of complete separation between segments. The outer cells undergo amesenchymal–epithelial transitionto form anepitheliumaround each somite. The inner cells remain asmesenchyme.
Notch signalling
editThe Notch system, as part of the clock and wavefront model, forms the boundaries of the somites.DLL1andDLL3are Notchligands,mutations of which cause various defects. Notch regulatesHES1,which sets up the caudal half of the somite. Notch activation turns onLFNGwhich in turn inhibits the Notch receptor. Notch activation also turns on the HES1 gene which inactivates LFNG, re-enabling the Notch receptor, and thus accounting for the oscillating clock model.MESP2induces theEPHA4gene, which causes repulsive interaction that separates somites by causing segmentation. EPHA4 is restricted to the boundaries of somites.EPHB2is also important for boundaries.
Mesenchymal-epithelial transition
editFibronectinandN-cadherinare key to the mesenchymal–epithelial transition process in the developing embryo. The process is probably regulated by paraxis and MESP2. In turn, MESP2 is regulated by Notch signaling. Paraxis is regulated by processes involving thecytoskeleton.
Specification
editTheHox genesspecify somites as a whole based on their position along the anterior-posterior axis through specifying the pre-somitic mesoderm before somitogenesis occurs. After somites are made, their identity as a whole has already been determined, as is shown by the fact that transplantation of somites from one region to a completely different region results in the formation of structures usually observed in the original region. In contrast, the cells within each somite retain plasticity (the ability to form any kind of structure) until relatively late in somitic development.[4]
Derivatives
editIn the developing vertebrateembryo,somites split to form dermatomes, skeletal muscle (myotomes),tendonsand cartilage (syndetomes)[9]andbone(sclerotomes).
Because the sclerotome differentiates before the dermatome and the myotome, the termdermomyotomerefers to the combined dermatome and myotome before they separate out.[10]
Dermatome
editThedermatomeis the dorsal portion of the paraxial mesoderm somite which gives rise to the skin (dermis). In the human embryo, it arises in the third week ofembryogenesis.[2]It is formed when a dermomyotome (the remaining part of the somite left when the sclerotome migrates), splits to form the dermatome and the myotome.[2]The dermatomes contribute to the skin,fatandconnective tissueof theneckand of the trunk, though most of the skin is derived fromlateral plate mesoderm.[2]
Myotome
editThemyotomeis that part of a somite that forms the muscles of the animal.[2]Each myotome divides into anepaxialpart (epimere), at the back, and a hypaxial part (hypomere) at the front.[2]Themyoblastsfrom the hypaxial division form the muscles of the thoracic and anterior abdominal walls. The epaxial muscle mass loses its segmental character to form theextensor musclesof the neck and trunk of mammals.
In fishes, salamanders, caecilians, and reptiles, the body musculature remains segmented as in the embryo, though it often becomes folded and overlapping, with epaxial and hypaxial masses divided into several distinct muscle groups.[citation needed]
Sclerotome
editThesclerotome(orcutis plate) forms thevertebraeand the rib cartilage and part of the occipital bone; the myotome forms themusculatureof the back, the ribs and the limbs; the syndetome forms the tendons and the dermatome forms theskinon the back. In addition, the somites specify the migration paths ofneural crestcells and theaxonsofspinal nerves.From their initial location within the somite, the sclerotome cells migrate medially towards thenotochord.These cells meet the sclerotome cells from the other side to form the vertebral body. The lower half of one sclerotome fuses with the upper half of the adjacent one to form each vertebral body.[11]From this vertebral body, sclerotome cells move dorsally and surround the developingspinal cord,forming the vertebral arch. Other cells move distally to the costal processes ofthoracic vertebraeto form the ribs.[11]
In arthropods
editIncrustaceandevelopment, a somite is a segment of the hypothetical primitive crustacean body plan. In current crustaceans, several of those somites may be fused.[12][13]
See also
editReferences
edit- ^Cuschieri, Alfred."The Third Week Of Life".University of Malta.Retrieved2007-10-13.
- ^abcdefLarsen, William J. (2001).Human embryology(3. ed.). Philadelphia, Pa.: Churchill Livingstone. pp. 53–86.ISBN978-0-443-06583-5.
- ^"Metamere".Dictionary and Thesaurus-Merriam-Webster Online.Merriam-Webster. 2012.Retrieved11 December2012.
- ^abcdGilbert, S.F. (2010).Developmental Biology(9th ed.). Sinauer Associates, Inc. pp.413–415.ISBN978-0-87893-384-6.
- ^Baker, R. E.;Schnell, S.;Maini, P. K.(2006)."A clock and wavefront mechanism for somite formation".Developmental Biology.293(1): 116–126.doi:10.1016/j.ydbio.2006.01.018.PMID16546158.
- ^Goldbeter, A.; Pourquié, O. (2008)."Modeling the segmentation clock as a network of coupled oscillations in the Notch, Wnt and FGF signaling pathways"(PDF).Journal of Theoretical Biology.252(3): 574–585.Bibcode:2008JThBi.252..574G.doi:10.1016/j.jtbi.2008.01.006.PMID18308339– viaUniversité libre de Bruxelles.
- ^Wahi, Kanu (2016). "The many roles of Notch signaling during vertebrate somitogenesis".Seminars in Cell and Developmental Biology.49:68–75.doi:10.1016/j.semcdb.2014.11.010.PMID25483003.S2CID10822545.
- ^Gomez, C; et al. (2008). "Control of segment number in vertebrate embryos".Nature.454(7202): 335–339.Bibcode:2008Natur.454..335G.doi:10.1038/nature07020.PMID18563087.S2CID4373389.
- ^Brent AE, Schweitzer R, Tabin CJ (April 2003)."A somitic compartment of tendon progenitors".Cell.113(2): 235–48.doi:10.1016/S0092-8674(03)00268-X.PMID12705871.S2CID16291509.
- ^"Embryo Images".University of North Carolina School of Medicine.Retrieved2007-10-19.
- ^ab Walker, Warren F., Jr. (1987)Functional Anatomy of the VertebrateSan Francisco: Saunders College Publishing.
- ^Ferrari, Frank D.; Fornshell, John; Vagelli, Alejandro A.; Ivanenko, V. N.; Dahms, Hans-Uwe (2011)."Early Post-Embryonic Development of Marine Chelicerates and Crustaceans with a Nauplius"(PDF).Crustaceana.84(7): 869–893.ISSN0011-216X.
- ^Manning, Raymond (1998)."A new genus and species of pinnotherid crab (Crustacea, Decapoda, Brachyura) from Indonesia".Zoosystema.20(2): 357–362.