Thecerebral cortex,also known as thecerebral mantle,[1]is the outer layer ofneural tissueof thecerebrumof thebraininhumansand othermammals.It is the largest site ofneural integrationin thecentral nervous system,[2]and plays a key role inattention,perception,awareness,thought,memory,language,andconsciousness.The cerebral cortex is the part of the brain responsible forcognition.
| Cerebral cortex | |
|---|---|
 | |
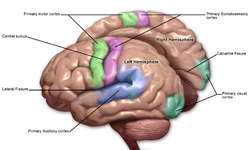 Motor and sensory areas of the cerebral cortex | |
| Details | |
| Part of | Cerebrum |
| Identifiers | |
| Latin | cortex cerebri |
| MeSH | D002540 |
| NeuroNames | 39 |
| NeuroLexID | birnlex_1494 |
| TA98 | A14.1.09.003 A14.1.09.301 |
| TA2 | 5527,5528 |
| FMA | 61830 |
| Anatomical terms of neuroanatomy | |
The six-layeredneocortexmakes up approximately 90% of thecortex,with theallocortexmaking up the remainder.[3]The cortex is divided into left and right parts by thelongitudinal fissure,which separates the twocerebral hemispheresthat are joined beneath the cortex by thecorpus callosum.In most mammals, apart from small mammals that have small brains, the cerebral cortex is folded, providing a greater surface area in the confined volume of thecranium.Apart from minimising brain and cranial volume,cortical foldingis crucial for thebrain circuitryand its functional organisation.[4]In mammals with small brains, there is no folding and the cortex is smooth.[5][6]
A fold or ridge in the cortex is termed agyrus(plural gyri) and a groove is termed asulcus(plural sulci). These surface convolutions appear duringfetal developmentand continue to mature after birth through the process ofgyrification.In thehuman brain,the majority of the cerebral cortex is not visible from the outside, but buried in the sulci.[7]The major sulci and gyri mark the divisions of the cerebrum into thelobes of the brain.The four major lobes are thefrontal,parietal,occipitalandtemporallobes. Other lobes are thelimbic lobe,and theinsular cortexoften referred to as theinsular lobe.
There are between 14 and 16 billionneuronsin the human cerebral cortex.[2]These are organised into horizontal cortical layers, and radially intocortical columnsandminicolumns.Cortical areas have specific functions such as movement in themotor cortex,and sight in thevisual cortex.The motor cortex is primarily located in theprecentral gyrus,and the visual cortex is located in the occipital lobe.
Structure
editThe cerebral cortex is the outer covering of the surfaces of the cerebral hemispheres and is folded into peaks calledgyri,and grooves calledsulci.In thehuman brain,it is between 2 and 3-4 mm. thick,[8]and makes up 40% of the brain's mass.[2]90% of the cerebral cortex is the six-layeredneocortexwhilst the other 10% is made up of the three/four-layeredallocortex.[2]There are between 14 and 16 billion neurons in the cortex.[2]These cortical neurons are organized radially incortical columns,andminicolumns,in the horizontally organized layers of the cortex.[9][10]
The neocortex is separable into different regions of cortex known in the plural as cortices, and include themotor cortexandvisual cortex.About two thirds of the cortical surface is buried in the sulci and theinsular cortexis completely hidden. The cortex is thickest over the top of a gyrus and thinnest at the bottom of a sulcus.[11]
Folds
editThe cerebral cortex is folded in a way that allows a large surface area ofneural tissueto fit within the confines of theneurocranium.When unfolded in the human, eachhemisphericcortex has a total surface area of about 0.12 square metres (1.3 sq ft).[12]The folding is inward away from the surface of the brain, and is also present on the medial surface of each hemisphere within thelongitudinal fissure.Most mammals have a cerebral cortex that is convoluted with the peaks known as gyri and the troughs or grooves known as sulci. Some small mammals including some smallrodentshave smooth cerebral surfaces withoutgyrification.[6]
Lobes
editThe larger sulci and gyri mark the divisions of the cortex of the cerebrum into thelobes of the brain.[8]There are four main lobes: thefrontal lobe,parietal lobe,temporal lobe,andoccipital lobe.Theinsular cortexis often included as the insular lobe.[13]Thelimbic lobeis a rim of cortex on the medial side of each hemisphere and is also often included.[14]There are also three lobules of the brain described: theparacentral lobule,thesuperior parietal lobule,and theinferior parietal lobule.
Thickness
editFor species of mammals, larger brains (in absolute terms, not just in relation to body size) tend to have thicker cortices.[15]The smallest mammals, such asshrews,have a neocortical thickness of about 0.5 mm; the ones with the largest brains, such as humans and fin whales, have thicknesses of 2–4 mm.[2][8]There is an approximatelylogarithmicrelationship between brain weight and cortical thickness.[15] Magnetic resonance imaging of the brain(MRI) makes it possible to get a measure for the thickness of the human cerebral cortex and relate it to other measures. The thickness of different cortical areas varies but in general, sensory cortex is thinner than motor cortex.[16]One study has found some positive association between the cortical thickness andintelligence.[17] Another study has found that thesomatosensory cortexis thicker inmigrainepatients, though it is not known if this is the result of migraine attacks, the cause of them or if both are the result of a shared cause.[18][19] A later study using a larger patient population reports no change in the cortical thickness in patients with migraine.[20] A genetic disorder of the cerebral cortex, whereby decreased folding in certain areas results in amicrogyrus,where there are four layers instead of six, is in some instances seen to be related todyslexia.[21]
Layers of neocortex
editTheneocortexis formed of six layers, numbered I to VI, from the outermost layer I – near to thepia mater,to the innermost layer VI – near to the underlyingwhite matter.Each cortical layer has a characteristic distribution of different neurons and their connections with other cortical and subcortical regions. There are direct connections between different cortical areas and indirect connections via the thalamus.
One of the clearest examples ofcortical layeringis theline of Gennariin theprimary visual cortex.This is a band of whiter tissue that can be observed with the naked eye in thecalcarine sulcusof the occipital lobe. The line of Gennari is composed ofaxonsbringing visual information from thethalamusinto layer IV of thevisual cortex.
Stainingcross-sections of the cortex to reveal the position of neuronal cell bodies and the intracortical axon tracts allowed neuroanatomists in the early 20th century to produce a detailed description of thelaminar structure of the cortexin different species. The work ofKorbinian Brodmann(1909) established that the mammalian neocortex is consistently divided into six layers.
Layer I
editLayer I is themolecular layer,and contains few scattered neurons, includingGABAergicrosehip neurons.[22]Layer I consists largely of extensions of apicaldendritictufts ofpyramidal neuronsand horizontally oriented axons, as well asglial cells.[4]During development,Cajal–Retzius cells[23]and subpial granular layer cells[24]are present in this layer. Also, some spinystellate cellscan be found here. Inputs to the apical tufts are thought to be crucial for thefeedbackinteractions in the cerebral cortex involved in associative learning and attention.[25]
While it was once thought that the input to layer I came from the cortex itself,[26]it is now known that layer I across the cerebral cortex receives substantial input frommatrixor M-type thalamus cells,[27]as opposed tocoreor C-type that go to layer IV.[28]
It is thought that layer I serves as a central hub for collecting and processing widespread information. It integrates ascending sensory inputs with top-down expectations, regulating how sensory perceptions align with anticipated outcomes. Further, layer I sorts, directs, and combines excitatory inputs, integrating them with neuromodulatory signals. Inhibitory interneurons, both within layer I and from other cortical layers, gate these signals. Together, these interactions dynamically calibrate information flow throughout the neocortex, shaping perceptions and experiences.[29]
Layer II
editLayer II, theexternal granular layer,contains smallpyramidal neuronsand numerous stellate neurons.
Layer III
editLayer III, theexternal pyramidal layer,contains predominantly small and medium-size pyramidal neurons, as well as non-pyramidal neurons with vertically oriented intracortical axons; layers I through III are the main target ofcommissuralcorticocorticalafferents,and layer III is the principal source of corticocorticalefferents.
Layer IV
editLayer IV, theinternal granular layer,contains different types ofstellateand pyramidal cells, and is the main target ofthalamocortical afferentsfrom thalamus type C neurons (core-type)[28]as well as intra-hemispheric corticocortical afferents. The layers above layer IV are also referred to as supragranular layers (layers I-III), whereas the layers below are referred to as infragranular layers (layers V and VI).African elephants,cetaceans,andhippopotamusdo not have a layer IV with axons which would terminate there going instead to the inner part of layer III.[30]
Layer V
editLayer V, theinternal pyramidal layer,contains large pyramidal neurons. Axons from these leave the cortex and connect with subcortical structures including thebasal ganglia.In the primary motor cortex of the frontal lobe, layer V contains giant pyramidal cells calledBetz cells,whose axons travel through theinternal capsule,thebrain stem,and the spinal cord forming thecorticospinal tract,which is the main pathway for voluntary motor control.
Layer VI
editLayer VI, thepolymorphic layerormultiform layer,contains few large pyramidal neurons and many small spindle-like pyramidal and multiform neurons; layer VI sendsefferent fibersto the thalamus, establishing a very precise reciprocal interconnection between the cortex and the thalamus.[31]That is, layer VI neurons from one cortical column connect with thalamus neurons that provide input to the same cortical column. These connections are both excitatory and inhibitory. Neurons sendexcitatoryfibers to neurons in the thalamus and also send collaterals to thethalamic reticular nucleusthatinhibitthese same thalamus neurons or ones adjacent to them.[32]One theory is that because the inhibitory output is reduced bycholinergicinput to the cerebral cortex, this provides thebrainstemwith adjustable "gain control for the relay oflemniscalinputs ".[32]
Columns
editThe cortical layers are not simply stacked one over the other; there exist characteristic connections between different layers and neuronal types, which span all the thickness of the cortex. These cortical microcircuits are grouped intocortical columnsandminicolumns.[33]It has been proposed that the minicolumns are the basic functional units of the cortex.[34]In 1957,Vernon Mountcastleshowed that the functional properties of the cortex change abruptly between laterally adjacent points; however, they are continuous in the direction perpendicular to the surface. Later works have provided evidence of the presence of functionally distinct cortical columns in the visual cortex (Hubel andWiesel,1959),[35]auditory cortex, and associative cortex.
Cortical areas that lack a layer IV are calledagranular.Cortical areas that have only a rudimentary layer IV are called dysgranular.[36]Information processing within each layer is determined by different temporal dynamics with that in layers II/III having a slow 2Hzoscillationwhile that in layer V has a fast 10–15 Hz oscillation.[37]
Types of cortex
editBased on the differences inlaminar organizationthe cerebral cortex can be classified into two types, the large area ofneocortexwhich has six cell layers, and the much smaller area ofallocortexthat has three or four layers:[3]
- The neocortex is also known as the isocortex or neopallium and is the part of the mature cerebral cortex with six distinct layers. Examples of neocortical areas include the granularprimary motor cortex,and the striateprimary visual cortex.The neocortex has two subtypes, thetrue isocortexand theproisocortexwhich is a transitional region between the isocortex and the regions of the periallocortex.
- The allocortex is the part of the cerebral cortex with three or four layers, and has three subtypes, thepaleocortexwith three cortical laminae, thearchicortexwhich has four or five, and a transitional area adjacent to the allocortex, theperiallocortex.Examples of allocortex are theolfactory cortexand thehippocampus.
There is a transitional area between the neocortex and the allocortex called theparalimbic cortex,where layers 2, 3 and 4 are merged. This area incorporates the proisocortex of the neocortex and the periallocortex of the allocortex. In addition, the cerebral cortex may be classified into fourlobes:thefrontal lobe,temporal lobe,theparietal lobe,and theoccipital lobe,named from their overlying bones of the skull.
Blood supply and drainage
editBlood supply to the cerebral cortex is part of thecerebral circulation.Cerebral arteriessupply the blood thatperfusesthe cerebrum. This arterial blood carries oxygen, glucose, and other nutrients to the cortex.Cerebral veinsdrain the deoxygenated blood, and metabolic wastes including carbon dioxide, back to the heart.
The main arteries supplying the cortex are theanterior cerebral artery,themiddle cerebral artery,and theposterior cerebral artery.The anterior cerebral artery supplies the anterior portions of the brain, including most of the frontal lobe. The middle cerebral artery supplies the parietal lobes, temporal lobes, and parts of the occipital lobes. The middle cerebral artery splits into two branches to supply the left and right hemisphere, where they branch further. The posterior cerebral artery supplies the occipital lobes.
Thecircle of Willisis the main blood system that deals with blood supply in the cerebrum and cerebral cortex.
Development
editTheprenatal developmentof the cerebral cortex is a complex and finely tuned process calledcorticogenesis,influenced by the interplay between genes and the environment.[38]
Neural tube
editThe cerebral cortex develops from the most anterior part, the forebrain region, of theneural tube.[39][40]Theneural platefolds and closes to form theneural tube.From the cavity inside the neural tube develops theventricular system,and, from theneuroepithelial cellsof its walls, theneuronsandgliaof the nervous system. The most anterior (front, or cranial) part of the neural plate, theprosencephalon,which is evident beforeneurulationbegins, gives rise to the cerebral hemispheres and later cortex.[41]
Cortical neuron development
editCortical neurons are generated within theventricular zone,next to theventricles.At first, this zone containsneural stem cells,that transition toradial glial cells–progenitor cells, which divide to produce glial cells and neurons.[42]
Radial glia
editThe cerebral cortex is composed of a heterogenous population of cells that give rise to different cell types. The majority of these cells are derived fromradial gliamigration that form the different cell types of the neocortex and it is a period associated with an increase inneurogenesis.Similarly, the process of neurogenesis regulates lamination to form the different layers of the cortex. During this process there is an increase in the restriction of cell fate that begins with earlierprogenitorsgiving rise to any cell type in the cortex and later progenitors giving rise only toneuronsof superficial layers. This differential cell fate creates an inside-out topography in the cortex with younger neurons in superficial layers and older neurons in deeper layers. In addition, laminar neurons are stopped inSorG2 phasein order to give a fine distinction between the different cortical layers. Laminar differentiation is not fully complete until after birth since during development laminar neurons are still sensitive to extrinsic signals and environmental cues.[43]
Although the majority of the cells that compose the cortex are derived locally from radial glia there is a subset population of neurons thatmigratefrom other regions. Radial glia give rise to neurons that are pyramidal in shape and useglutamateas aneurotransmitter,however these migrating cells contribute neurons that are stellate-shaped and useGABAas their main neurotransmitter. These GABAergic neurons are generated by progenitor cells in themedial ganglionic eminence(MGE) that migrate tangentially to the cortex via thesubventricular zone.This migration of GABAergic neurons is particularly important sinceGABA receptorsare excitatory during development. This excitation is primarily driven by the flux of chloride ions through the GABA receptor, however in adults chloride concentrations shift causing an inward flux of chloride thathyperpolarizespostsynaptic neurons.[44] The glial fibers produced in the first divisions of the progenitor cells are radially oriented, spanning the thickness of the cortex from theventricular zoneto the outer,pialsurface, and provide scaffolding for the migration of neurons outwards from theventricular zone.[45][46]
At birth there are very fewdendritespresent on the cortical neuron's cell body, and the axon is undeveloped. During the first year of life the dendrites become dramatically increased in number, such that they can accommodate up to a hundred thousandsynaptic connectionswith other neurons. The axon can develop to extend a long way from the cell body.[47]
Asymmetric division
editThe first divisions of the progenitor cells are symmetric, which duplicates the total number of progenitor cells at eachmitotic cycle.Then, some progenitor cells begin to divide asymmetrically, producing one postmitotic cell that migrates along the radial glial fibers, leaving theventricular zone,and one progenitor cell, which continues to divide until the end of development, when it differentiates into aglial cellor anependymal cell.As theG1 phaseofmitosisis elongated, in what is seen as selective cell-cycle lengthening, the newly born neurons migrate to more superficial layers of the cortex.[48]The migrating daughter cells become thepyramidal cellsof the cerebral cortex.[49]The development process is time ordered and regulated by hundreds of genes andepigenetic regulatory mechanisms.[50]
Layer organization
editThelayered structureof the mature cerebral cortex is formed during development. The first pyramidal neurons generated migrate out of theventricular zoneandsubventricular zone,together withreelin-producingCajal–Retzius neurons,from thepreplate.Next, a cohort of neurons migrating into the middle of the preplate divides this transient layer into the superficialmarginal zone,which will become layer I of the mature neocortex, and thesubplate,[51]forming a middle layer called thecortical plate.These cells will form the deep layers of the mature cortex, layers five and six. Later born neurons migrate radially into the cortical plate past the deep layer neurons, and become the upper layers (two to four). Thus, the layers of the cortex are created in an inside-out order.[52]The only exception to this inside-out sequence ofneurogenesisoccurs in the layer I ofprimates,in which, in contrast torodents,neurogenesis continues throughout the entire period ofcorticogenesis.[53]
Cortical patterning
editThe map of functional cortical areas, which include primary motor and visual cortex, originates from a 'protomap',[54]which is regulated by molecular signals such asfibroblast growth factorFGF8early in embryonic development.[55][56]These signals regulate the size, shape, and position of cortical areas on the surface of the cortical primordium, in part by regulating gradients oftranscription factorexpression, through a process calledcortical patterning.Examples of such transcription factors include the genesEMX2andPAX6.[57]Together, bothtranscription factorsform an opposing gradient of expression.Pax6is highly expressed at therostral lateralpole, whileEmx2is highly expressed in thecaudomedialpole. The establishment of this gradient is important for proper development. For example,mutationsin Pax6 can cause expression levels of Emx2 to expand out of its normal expression domain, which would ultimately lead to an expansion of the areas normally derived from the caudal medial cortex, such as thevisual cortex.On the contrary, if mutations in Emx2 occur, it can cause the Pax6-expressing domain to expand and result in thefrontalandmotor corticalregions enlarging. Therefore, researchers believe that similar gradients andsignaling centersnext to the cortex could contribute to the regional expression of these transcription factors.[44] Two very well studied patterning signals for the cortex includeFGFandretinoic acid.If FGFs aremisexpressedin different areas of the developing cortex,cortical patterningis disrupted. Specifically, whenFgf8is increased in theanteriorpole, Emx2 isdownregulatedand acaudalshift in the cortical region occurs. This ultimately causes an expansion of the rostral regions. Therefore, Fgf8 and other FGFs play a role in the regulation of expression of Emx2 and Pax6 and represent how the cerebral cortex can become specialized for different functions.[44]
Rapid expansion of the cortical surface area is regulated by the amount of self-renewal ofradial glial cellsand is partly regulated byFGFandNotch genes.[58]During the period of cortical neurogenesis and layer formation, many higher mammals begin the process ofgyrification,which generates the characteristic folds of the cerebral cortex.[59][60]Gyrification is regulated by a DNA-associated proteinTrnp1[61]and by FGF andSHHsignaling.[62][63]
Evolution
editOf all the different brain regions, the cerebral cortex shows the largest evolutionary variation and has evolved most recently.[6]In contrast to the highly conserved circuitry of themedulla oblongata,for example, which serves critical functions such as regulation of heart and respiration rates, many areas of the cerebral cortex are not strictly necessary for survival. Thus, the evolution of the cerebral cortex has seen the advent and modification of new functional areas—particularly association areas that do not directly receive input from outside the cortex.[6]
A key theory of cortical evolution is embodied in theradial unit hypothesisand relatedprotomaphypothesis, first proposed by Rakic.[64]This theory states that new cortical areas are formed by the addition of new radial units, which is accomplished at thestem celllevel. The protomap hypothesis states that the cellular and molecular identity and characteristics of neurons in each cortical area are specified by corticalstem cells,known asradial glial cells,in a primordial map. This map is controlled by secreted signalingproteinsand downstreamtranscription factors.[65][66][67]
Function
editConnections
editThe cerebral cortex is connected to various subcortical structures such as thethalamusand thebasal ganglia,sending information to them alongefferent connectionsand receiving information from them viaafferent connections.Most sensory information is routed to the cerebral cortex via the thalamus. Olfactory information, however, passes through theolfactory bulbto the olfactory cortex (piriform cortex). The majority of connections are from one area of the cortex to another, rather than from subcortical areas;Braitenbergand Schüz (1998) claim that in primary sensory areas, at the cortical level where the input fibers terminate, up to 20% of the synapses are supplied by extracortical afferents but that in other areas and other layers the percentage is likely to be much lower.[68]
Cortical areas
editThe whole of the cerebral cortex was divided into 52 different areas in an early presentation byKorbinian Brodmann.These areas, known asBrodmann areas,are based on theircytoarchitecturebut also relate to various functions. An example is Brodmann area 17, which is theprimary visual cortex.
In more general terms the cortex is typically described as comprising three parts: sensory, motor, and association areas.
Sensory areas
editThe sensory areas are the cortical areas that receive and process information from thesenses.Parts of the cortex that receive sensory inputs from thethalamusare called primary sensory areas. The senses of vision, hearing, and touch are served by the primary visual cortex, primaryauditory cortexandprimary somatosensory cortexrespectively. In general, the two hemispheres receive information from the opposite (contralateral) side of thebody.For example, the right primary somatosensory cortex receives information from the left limbs, and the right visual cortex receives information from the left visualfield.
The organization of sensory maps in the cortex reflects that of the corresponding sensing organ, in what is known as atopographic map.Neighboring points in the primaryvisual cortex,for example, correspond to neighboring points in theretina.This topographic map is called aretinotopic map.In the same way, there exists atonotopic mapin the primary auditory cortex and asomatotopic mapin the primary sensory cortex. This last topographic map of the body onto theposterior central gyrushas been illustrated as a deformed human representation, the somatosensoryhomunculus,where the size of different body parts reflects the relative density of their innervation. Areas with much sensory innervation, such as the fingertips and the lips, require more cortical area to process finer sensation.
Motor areas
editThe motor areas are located in both hemispheres of the cortex. The motor areas are very closely related to the control of voluntary movements, especially fine fragmented movements performed by the hand. The right half of the motor area controls the left side of the body, and vice versa.
Two areas of the cortex are commonly referred to as motor:
- Primary motor cortex,whichexecutesvoluntary movements[69]
- Supplementary motor areasandpremotor cortex,whichselectvoluntary movements.[70]
In addition, motor functions have been described for:
- Posterior parietal cortex,which guides voluntary movements in space
- Dorsolateral prefrontal cortex,which decides which voluntary movements to make according to higher-order instructions, rules, and self-generated thoughts.
Just underneath the cerebral cortex are interconnected subcortical masses of grey matter calledbasal ganglia(or nuclei). The basal ganglia receive input from the substantia nigra of the midbrain and motor areas of the cerebral cortex, and send signals back to both of these locations. They are involved in motor control. They are found lateral to the thalamus. The main components of the basal ganglia are thecaudate nucleus,theputamen,theglobus pallidus,thesubstantia nigra,thenucleus accumbens,and thesubthalamic nucleus.The putamen and globus pallidus are also collectively known as thelentiform nucleus,because together they form a lens-shaped body. The putamen and caudate nucleus are also collectively called thecorpus striatumafter their striped appearance.[71][72]
Association areas
editThe association areas are the parts of the cerebral cortex that do not belong to the primary regions. They function to produce a meaningfulperceptual experienceof the world, enable us to interact effectively, and support abstract thinking and language. Theparietal,temporal,andoccipital lobes– all located in the posterior part of the cortex – integrate sensory information and information stored in memory. Thefrontal lobeor prefrontal association complex is involved in planning actions and movement, as well as abstract thought. Globally, the association areas are organized as distributed networks.[73]Each network connects areas distributed across widely spaced regions of the cortex. Distinct networks are positioned adjacent to one another yielding a complex series of interwoven networks. The specific organization of the association networks is debated with evidence for interactions, hierarchical relationships, and competition between networks.
In humans, association networks are particularly important to language function. In the past it was theorized that language abilities are localized inBroca's areain areas of the leftinferior frontal gyrus,BA44andBA45,for language expression and inWernicke's areaBA22,for language reception. However, the processes of language expression and reception have been shown to occur in areas other than just those structures around thelateral sulcus,including the frontal lobe,basal ganglia,cerebellum,andpons.[74]
Clinical significance
editNeurodegenerative diseasessuch asAlzheimer's disease,show as a marker, an atrophy of the grey matter of the cerebral cortex.[76]
Otherdiseases of the central nervous systemincludeneurological disorderssuch asepilepsy,movement disorders,anddifferent types of aphasia(difficulties in speech expression or comprehension).
Brain damagefrom disease or trauma, can involve damage to a specific lobe such as infrontal lobe disorder,and associated functions will be affected. Theblood–brain barrierthat serves to protect the brain from infection can become compromised allowing entry topathogens.
Thedeveloping fetusis susceptible to a range of environmental factors that can causebirth defectsand problems in later development. Maternal alcohol consumption for example can causefetal alcohol spectrum disorder.[77]Other factors that can cause neurodevelopment disorders aretoxicantssuch asdrugs,and exposure toradiationas fromX-rays.Infections can also affect the development of the cortex. A viral infection is one of the causes oflissencephaly,which results in a smooth cortex withoutgyrification.
A type ofelectrocorticographycalledcortical stimulation mappingis an invasive procedure that involves placingelectrodesdirectly onto the exposed brain in order to localise the functions of specific areas of the cortex. It is used in clinical and therapeutic applications including pre-surgical mapping.[78]
Genes associated with cortical disorders
editThere are a number of genetic mutations that can cause a wide range ofgenetic disordersof the cerebral cortex, includingmicrocephaly,schizencephalyand types oflissencephaly.[79]Chromosome abnormalitiescan also result causing a number ofneurodevelopmental disorderssuch asfragile X syndromeandRett syndrome.
MCPH1codes formicrocephalin,and disorders in this and inASPMare associated with microcephaly.[79]Mutations in the geneNBS1that codes fornibrincan causeNijmegen breakage syndrome,characterised by microcephaly.[79]
Mutations inEMX2,[80]andCOL4A1are associated withschizencephaly,[81]a condition marked by the absence of large parts of the cerebral hemispheres.
History
editIn 1909,Korbinian Brodmanndistinguished 52 different regions of the cerebral cortex based on their cytoarchitecture. These are known asBrodmann areas.[82]
Rafael Lorente de Nó,a student ofSantiago Ramon y Cajal,identified more than 40 different types of cortical neurons based on the distribution of their dendrites and axons.[82]
Other animals
editThe cerebral cortex is derived from thepallium,a layered structure found in theforebrainof allvertebrates.The basic form of the pallium is a cylindrical layer enclosing fluid-filled ventricles. Around the circumference of the cylinder are four zones, the dorsal pallium, medial pallium, ventral pallium, and lateral pallium, which are thought to behomologousto theneocortex,hippocampus,amygdala,andolfactory cortex,respectively.
Inavian brains,evidence suggests theavian pallium's neuroarchitecture to be reminiscent of the mammalian cerebral cortex.[83]The avian pallium has also been suggested to be an equivalent neural basis forconsciousness.[84][85]
Until recently no counterpart to the cerebral cortex had been recognized in invertebrates. However, a study published in the journalCellin 2010, based on gene expression profiles, reported strong affinities between the cerebral cortex and themushroom bodiesof theragwormPlatynereis dumerilii.[86]Mushroom bodies are structures in the brains of many types of worms and arthropods that are known to play important roles in learning and memory; the genetic evidence indicates a common evolutionary origin, and therefore indicates that the origins of the earliest precursors of the cerebral cortex date back to thePrecambrianera.
Additional images
edit-
Lateral surface of the human cerebral cortex
-
Medial surface of the human cerebral cortex
-
Tissue slice from the brain of an adultmacaquemonkey. The cerebral cortex is depicted in dark violet.
See also
editReferences
edit- ^"cerebral mantle".TheFreeDictionary.com.Retrieved9 May2024.
- ^abcdefSaladin K (2011).Human anatomy(3rd ed.). McGraw-Hill. pp. 416–422.ISBN978-0-07-122207-5.
- ^abStrominger NL, Demarest RJ, Laemle LB (2012). "Cerebral Cortex".Noback's Human Nervous System(Seventh ed.). Humana Press. pp. 429–451.doi:10.1007/978-1-61779-779-8_25.ISBN978-1-61779-778-1.
- ^abShipp S (June 2007)."Structure and function of the cerebral cortex".Current Biology.17(12): R443–R449.Bibcode:2007CBio...17.R443S.doi:10.1016/j.cub.2007.03.044.PMC1870400.PMID17580069.
- ^Fernández V, Llinares-Benadero C, Borrell V (May 2016)."Cerebral cortex expansion and folding: what have we learned?".The EMBO Journal.35(10): 1021–1044.doi:10.15252/embj.201593701.PMC4868950.PMID27056680.
- ^abcdRakic P (October 2009)."Evolution of the neocortex: a perspective from developmental biology".Nature Reviews. Neuroscience.10(10): 724–735.doi:10.1038/nrn2719.PMC2913577.PMID19763105.
- ^Principles of neural science(4th ed.). McGraw-Hill, Health Professions Division. 5 January 2000.ISBN978-0-8385-7701-1.
- ^abcRoberts P (1992).Neuroanatomy(3rd ed.). Springer-Verlag. pp. 86–92.ISBN978-0-387-97777-5.
- ^Lodato S, Arlotta P (13 November 2015)."Generating neuronal diversity in the mammalian cerebral cortex".Annual Review of Cell and Developmental Biology.31(1): 699–720.doi:10.1146/annurev-cellbio-100814-125353.PMC4778709.PMID26359774.
Functional columns were first defined in the cortex by Mountcastle (1957), who proposed the columnar hypothesis, which states that the cortex is composed of discrete, modular columns of neurons, characterized by a consistent connectivity profile.
- ^Ansen-Wilson LJ, Lipinski RJ (January 2017)."Gene-environment interactions in cortical interneuron development and dysfunction: A review of preclinical studies".Neurotoxicology.58:120–129.Bibcode:2017NeuTx..58..120A.doi:10.1016/j.neuro.2016.12.002.PMC5328258.PMID27932026.
- ^Carpenter MB (1985).Core text of neuroanatomy(3rd ed.). Williams & Wilkins. pp. 348–358.ISBN978-0-683-01455-6.
- ^Toro R, Perron M, Pike B, Richer L, Veillette S, Pausova Z, et al. (October 2008)."Brain size and folding of the human cerebral cortex".Cerebral Cortex.18(10): 2352–2357.doi:10.1093/cercor/bhm261.PMID18267953.
- ^Nieuwenhuys R (2012). "The insular cortex".Evolution of the Primate Brain.Progress in Brain Research. Vol. 195. pp. 123–63.doi:10.1016/B978-0-444-53860-4.00007-6.ISBN978-0-444-53860-4.PMID22230626.
- ^Tortora G, Derrickson B (2011).Principles of anatomy & physiology(13th. ed.). Wiley. p. 549.ISBN978-0-470-64608-3.
- ^abNieuwenhuys R, Donkelaar HJ, Nicholson C (1998).The central nervous system of vertebrates, Volume 1.Springer. pp. 2011–2012.ISBN978-3-540-56013-5.
- ^Kruggel F, Brückner MK, Arendt T, Wiggins CJ, von Cramon DY (September 2003). "Analyzing the neocortical fine-structure".Medical Image Analysis.7(3): 251–264.doi:10.1016/S1361-8415(03)00006-9.hdl:11858/00-001M-0000-0010-9C60-3.PMID12946467.
- ^Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, et al. (September 2007)."Relationships between IQ and regional cortical gray matter thickness in healthy adults".Cerebral Cortex.17(9): 2163–2171.doi:10.1093/cercor/bhl125.PMID17118969.
- ^DaSilva AF, Granziera C, Snyder J, Hadjikhani N (November 2007)."Thickening in the somatosensory cortex of patients with migraine".Neurology.69(21): 1990–1995.doi:10.1212/01.wnl.0000291618.32247.2d.PMC3757544.PMID18025393.
- ^Paddock C (20 November 2007)."Migraine Sufferers Have Thicker Brain Cortex".Medical News Today.Archivedfrom the original on 11 May 2008.
- ^Datta R, Detre JA, Aguirre GK, Cucchiara B (October 2011)."Absence of changes in cortical thickness in patients with migraine".Cephalalgia.31(14): 1452–1458.doi:10.1177/0333102411421025.PMC3512201.PMID21911412.
- ^Habib M (December 2000)."The neurological basis of developmental dyslexia: an overview and working hypothesis".Brain.123 Pt 12 (12): 2373–2399.doi:10.1093/brain/123.12.2373.PMID11099442.
- ^"Scientists identify a new kind of human brain cell".Allen Institute.27 August 2018.
- ^Meyer G, Goffinet AM, Fairén A (December 1999). "What is a Cajal-Retzius cell? A reassessment of a classical cell type based on recent observations in the developing neocortex".Cerebral Cortex.9(8): 765–775.doi:10.1093/cercor/9.8.765.PMID10600995.
- ^Judaš M, Pletikos M (2010)."The discovery of the subpial granular layer in the human cerebral cortex".Translational Neuroscience.1(3): 255–260.doi:10.2478/v10134-010-0037-4.S2CID143409890.
- ^Gilbert CD, Sigman M (June 2007)."Brain states: top-down influences in sensory processing".Neuron.54(5): 677–696.doi:10.1016/j.neuron.2007.05.019.hdl:11336/67502.PMID17553419.
- ^Cauller L (November 1995). "Layer I of primary sensory neocortex: where top-down converges upon bottom-up".Behavioural Brain Research.71(1–2): 163–170.doi:10.1016/0166-4328(95)00032-1.PMID8747184.S2CID4015532.
- ^Rubio-Garrido P, Pérez-de-Manzo F, Porrero C, Galazo MJ, Clascá F (October 2009)."Thalamic input to distal apical dendrites in neocortical layer 1 is massive and highly convergent".Cerebral Cortex.19(10): 2380–2395.doi:10.1093/cercor/bhn259.PMID19188274.
- ^abJones EG (July 1998). "Viewpoint: the core and matrix of thalamic organization".Neuroscience.85(2): 331–345.doi:10.1016/S0306-4522(97)00581-2.PMID9622234.S2CID17846130.
- ^Huang S, Wu SJ, Sansone G, Ibrahim LA, Fishell G (January 2024). "Layer 1 neocortex: Gating and integrating multidimensional signals".Neuron.112(2): 184–200.doi:10.1016/j.neuron.2023.09.041.PMC11180419.PMID37913772.
- ^Bhagwandin A, Molnár Z, Bertelsen MF, Karlsson KÆ, Alagaili AN, Bennett NC, et al. (July 2024). "Where Do Core Thalamocortical Axons Terminate in Mammalian Neocortex When There Is No Cytoarchitecturally Distinct Layer 4?".The Journal of Comparative Neurology.532(7): e25652.doi:10.1002/cne.25652.PMID38962882.
- ^Creutzfeldt, O. 1995.Cortex Cerebri.Springer-Verlag.
- ^abLam YW, Sherman SM (January 2010)."Functional organization of the somatosensory cortical layer 6 feedback to the thalamus".Cerebral Cortex.20(1): 13–24.doi:10.1093/cercor/bhp077.PMC2792186.PMID19447861.
- ^Suzuki IK, Hirata T (January 2013)."Neocortical neurogenesis is not really" neo ": a new evolutionary model derived from a comparative study of chick pallial development".Development, Growth & Differentiation.55(1): 173–187.doi:10.1111/dgd.12020.PMID23230908.S2CID36706690.
- ^Mountcastle VB (April 1997)."The columnar organization of the neocortex".Brain.120 ( Pt 4) (4): 701–722.doi:10.1093/brain/120.4.701.PMID9153131.
- ^Hubel DH, Wiesel TN (October 1959)."Receptive fields of single neurones in the cat's striate cortex".The Journal of Physiology.148(3): 574–591.doi:10.1113/jphysiol.1959.sp006308.PMC1363130.PMID14403679.
- ^S.M. Dombrowski, C.C. Hilgetag, and H. Barbas.Quantitative Architecture Distinguishes Prefrontal Cortical Systems in the Rhesus MonkeyArchived2008-08-29 at theWayback Machine.Cereb.Cortex11: 975–988. "...they either lack (agranular) or have only a rudimentary granular layer IV (dysgranular)."
- ^Sun W, Dan Y (October 2009)."Layer-specific network oscillation and spatiotemporal receptive field in the visual cortex".Proceedings of the National Academy of Sciences of the United States of America.106(42): 17986–17991.Bibcode:2009PNAS..10617986S.doi:10.1073/pnas.0903962106.PMC2764922.PMID19805197.
- ^Pletikos M, Sousa AM, Sedmak G, Meyer KA, Zhu Y, Cheng F, et al. (January 2014)."Temporal specification and bilaterality of human neocortical topographic gene expression".Neuron.81(2): 321–332.doi:10.1016/j.neuron.2013.11.018.PMC3931000.PMID24373884.
- ^Wolpert L (2015).Principles of Development(Fifth ed.). UK: Oxford University Press. p. 533.ISBN978-0-19-967814-3.
- ^Warren N, Caric D, Pratt T, Clausen JA, Asavaritikrai P, Mason JO, et al. (September 1999)."The transcription factor, Pax6, is required for cell proliferation and differentiation in the developing cerebral cortex".Cerebral Cortex.9(6): 627–635.doi:10.1093/cercor/9.6.627.PMID10498281.
- ^Larsen WJ, Sherman LS, Potter SS, Scott WJ (2001).Human Embryology(3rd ed.). New York: Churchill Livingstone. pp. 421–422.ISBN978-0-443-06583-5.
- ^Noctor SC, Flint AC,Weissman TA,Dammerman RS, Kriegstein AR (February 2001). "Neurons derived from radial glial cells establish radial units in neocortex".Nature.409(6821): 714–720.Bibcode:2001Natur.409..714N.doi:10.1038/35055553.PMID11217860.S2CID3041502.
- ^Sur M, Leamey CA (April 2001). "Development and plasticity of cortical areas and networks".Nature Reviews. Neuroscience.2(4): 251–262.doi:10.1038/35067562.PMID11283748.S2CID893478.
- ^abcSanes DH, Reh TA, Harris WA (2012).Development of the Nervous System.Elsevier Inc.ISBN978-0-12-374539-2.
- ^Rakic P (October 2009)."Evolution of the neocortex: a perspective from developmental biology".Nature Reviews. Neuroscience.10(10): 724–735.doi:10.1038/nrn2719.PMC2913577.PMID19763105.
- ^Rakic P (November 1972). "Extrinsic cytological determinants of basket and stellate cell dendritic pattern in the cerebellar molecular layer".The Journal of Comparative Neurology.146(3): 335–354.doi:10.1002/cne.901460304.PMID4628749.S2CID31900267.
- ^Gilbert S (2006).Developmental Biology(8th ed.). Sinauer Associates Publishers. pp. 394–395.ISBN978-0-87893-250-4.
- ^Calegari F, Haubensak W, Haffner C, Huttner WB (July 2005)."Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development".The Journal of Neuroscience.25(28): 6533–6538.doi:10.1523/jneurosci.0778-05.2005.PMC6725437.PMID16014714.
- ^Rakic P (July 1988). "Specification of cerebral cortical areas".Science.241(4862): 170–176.Bibcode:1988Sci...241..170R.doi:10.1126/science.3291116.PMID3291116.
- ^Hu XL, Wang Y, Shen Q (April 2012)."Epigenetic control on cell fate choice in neural stem cells".Protein & Cell.3(4): 278–290.doi:10.1007/s13238-012-2916-6.PMC4729703.PMID22549586.
- ^Kostovic I, Rakic P (July 1990). "Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain".The Journal of Comparative Neurology.297(3): 441–470.doi:10.1002/cne.902970309.PMID2398142.S2CID21371568.
- ^Rakic P (February 1974). "Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition".Science.183(4123): 425–427.Bibcode:1974Sci...183..425R.doi:10.1126/science.183.4123.425.PMID4203022.S2CID10881759.
- ^Zecevic N, Rakic P (August 2001)."Development of layer I neurons in the primate cerebral cortex".The Journal of Neuroscience.21(15): 5607–5619.doi:10.1523/JNEUROSCI.21-15-05607.2001.PMC6762645.PMID11466432.
- ^Rakic P (July 1988). "Specification of cerebral cortical areas".Science.241(4862): 170–176.Bibcode:1988Sci...241..170R.doi:10.1126/science.3291116.PMID3291116.
- ^Fukuchi-Shimogori T, Grove EA (November 2001)."Neocortex patterning by the secreted signaling molecule FGF8".Science.294(5544): 1071–1074.Bibcode:2001Sci...294.1071F.doi:10.1126/science.1064252.PMID11567107.S2CID14807054.
- ^Garel S, Huffman KJ, Rubenstein JL (May 2003). "Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants".Development.130(9): 1903–1914.doi:10.1242/dev.00416.PMID12642494.S2CID6533589.
- ^Bishop KM, Goudreau G, O'Leary DD (April 2000). "Regulation of area identity in the mammalian neocortex by Emx2 and Pax6".Science.288(5464): 344–349.Bibcode:2000Sci...288..344B.doi:10.1126/science.288.5464.344.PMID10764649.
- ^Rash BG, Lim HD, Breunig JJ, Vaccarino FM (October 2011)."FGF signaling expands embryonic cortical surface area by regulating Notch-dependent neurogenesis".The Journal of Neuroscience.31(43): 15604–15617.doi:10.1523/jneurosci.4439-11.2011.PMC3235689.PMID22031906.
- ^Rajagopalan V, Scott J, Habas PA, Kim K, Corbett-Detig J, Rousseau F, et al. (February 2011)."Local tissue growth patterns underlying normal fetal human brain gyrification quantified in utero".The Journal of Neuroscience.31(8): 2878–2887.doi:10.1523/jneurosci.5458-10.2011.PMC3093305.PMID21414909.
- ^Lui JH, Hansen DV, Kriegstein AR (July 2011)."Development and evolution of the human neocortex".Cell.146(1): 18–36.doi:10.1016/j.cell.2011.06.030.PMC3610574.PMID21729779.
- ^Stahl R, Walcher T, De Juan Romero C, Pilz GA, Cappello S, Irmler M, et al. (April 2013)."Trnp1 regulates expansion and folding of the mammalian cerebral cortex by control of radial glial fate".Cell.153(3): 535–549.doi:10.1016/j.cell.2013.03.027.hdl:10261/338716.PMID23622239.
- ^Wang L, Hou S, Han YG (July 2016)."Hedgehog signaling promotes basal progenitor expansion and the growth and folding of the neocortex".Nature Neuroscience.19(7): 888–896.doi:10.1038/nn.4307.PMC4925239.PMID27214567.
- ^Rash BG, Tomasi S, Lim HD, Suh CY, Vaccarino FM (June 2013)."Cortical gyrification induced by fibroblast growth factor 2 in the mouse brain".The Journal of Neuroscience.33(26): 10802–10814.doi:10.1523/JNEUROSCI.3621-12.2013.PMC3693057.PMID23804101.
- ^Rakic P (July 1988). "Specification of cerebral cortical areas".Science.241(4862): 170–176.Bibcode:1988Sci...241..170R.doi:10.1126/science.3291116.PMID3291116.
- ^Fukuchi-Shimogori T, Grove EA (November 2001)."Neocortex patterning by the secreted signaling molecule FGF8".Science.294(5544): 1071–1074.Bibcode:2001Sci...294.1071F.doi:10.1126/science.1064252.PMID11567107.S2CID14807054.
- ^Bishop KM, Goudreau G, O'Leary DD (April 2000). "Regulation of area identity in the mammalian neocortex by Emx2 and Pax6".Science.288(5464): 344–349.Bibcode:2000Sci...288..344B.doi:10.1126/science.288.5464.344.PMID10764649.
- ^Grove EA, Fukuchi-Shimogori T (2003). "Generating the cerebral cortical area map".Annual Review of Neuroscience.26:355–380.doi:10.1146/annurev.neuro.26.041002.131137.PMID14527269.S2CID12282525.
- ^Braitenberg V, Schüz A (1998).Cortex: Statistics and Geometry of Neuronal Connectivity.New York: Springer Science & Business Media.ISBN978-3-540-63816-2.
- ^Moini J, Piran P (January 2020)."Chapter 6 - Cerebral cortex".In Moini J, Piran P (eds.).Functional and Clinical Neuroanatomy: A Guide for Health Care Professionals.Academic Press. pp. 177–240.doi:10.1016/B978-0-12-817424-1.00006-9.ISBN978-0-12-817424-1.
- ^Michelet T, Burbaud P, Gross CE, Bioulac B (January 2010)."Behavioral Planning: Neurophysiological Approach of the Frontal Lobe Function in Primates".In Koob GF, Le Moal M, Thompson RF (eds.).Encyclopedia of Behavioral Neuroscience.Academic Press. pp. 145–152.doi:10.1016/B978-0-08-045396-5.00213-X.ISBN978-0-08-045396-5.
- ^Saladin, Kenneth. Anatomy and Physiology: The Unity of Form and Function, 5th Ed. New York: McGraw-Hill Companies Inc., 2010. Print.
- ^Dorland's Medical Dictionary for Health Consumers, 2008.
- ^Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (September 2011)."The organization of the human cerebral cortex estimated by intrinsic functional connectivity".Journal of Neurophysiology.106(3): 1125–1165.doi:10.1152/jn.00338.2011.PMC3174820.PMID21653723.
- ^Price CJ (October 2000)."The anatomy of language: contributions from functional neuroimaging".Journal of Anatomy.197 Pt 3 (Pt 3): 335–359.doi:10.1046/j.1469-7580.2000.19730335.x.PMC1468137.PMID11117622.
- ^Kentar M, Mann M, Sahm F, Olivares-Rivera A, Sanchez-Porras R, Zerelles R, et al. (March 2020). "Detection of spreading depolarizations in a middle cerebral artery occlusion model in swine".Acta Neurochirurgica.162(3): 581–592.doi:10.1007/s00701-019-04132-8.PMID31940093.S2CID210196036.
- ^Nakazawa T, Ohara T, Hirabayashi N, Furuta Y, Hata J, Shibata M, et al. (March 2022)."Multiple-region grey matter atrophy as a predictor for the development of dementia in a community: the Hisayama Study".Journal of Neurology, Neurosurgery, and Psychiatry.93(3): 263–271.doi:10.1136/jnnp-2021-326611.PMC8862082.PMID34670843.
- ^Mukherjee RA, Hollins S, Turk J (June 2006)."Fetal alcohol spectrum disorder: an overview".Journal of the Royal Society of Medicine.99(6): 298–302.doi:10.1177/014107680609900616.PMC1472723.PMID16738372.
- ^Tarapore PE, Tate MC, Findlay AM, Honma SM, Mizuiri D, Berger MS, et al. (August 2012)."Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation".Journal of Neurosurgery.117(2): 354–362.doi:10.3171/2012.5.JNS112124.PMC4060619.PMID22702484.
- ^abcMochida GH, Walsh CA (May 2004). "Genetic basis of developmental malformations of the cerebral cortex".Archives of Neurology.61(5): 637–640.doi:10.1001/archneur.61.5.637.PMID15148137.
- ^"EMX2 empty spiracles homeobox 2 [Homo sapiens (human)]".Gene – NCBI.National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^Smigiel R, Cabala M, Jakubiak A, Kodera H, Sasiadek MJ, Matsumoto N, et al. (April 2016). "Novel COL4A1 mutation in an infant with severe dysmorphic syndrome with schizencephaly, periventricular calcifications, and cataract resembling congenital infection".Birth Defects Research. Part A, Clinical and Molecular Teratology.106(4): 304–307.doi:10.1002/bdra.23488.PMID26879631.
- ^abKendel ER, Mack S, eds. (2013).Principles of Neural Science(5th ed.). New York: McGraw-Hill Medical. pp. 347–348.ISBN978-0-07-139011-8.OCLC795553723.
- ^Stacho M, Herold C, Rook N, Wagner H, Axer M, Amunts K, et al. (September 2020). "A cortex-like canonical circuit in the avian forebrain".Science.369(6511).doi:10.1126/science.abc5534.PMID32973004.
- ^Nieder A, Wagener L, Rinnert P (September 2020). "A neural correlate of sensory consciousness in a corvid bird".Science.369(6511): 1626–1629.Bibcode:2020Sci...369.1626N.doi:10.1126/science.abb1447.PMID32973028.S2CID221881862.
- ^Herculano-Houzel S (September 2020). "Birds do have a brain cortex-and think".Science.369(6511): 1567–1568.Bibcode:2020Sci...369.1567H.doi:10.1126/science.abe0536.PMID32973020.S2CID221882004.
- ^Tomer R, Denes AS, Tessmar-Raible K, Arendt D (September 2010)."Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium".Cell.142(5): 800–809.doi:10.1016/j.cell.2010.07.043.PMID20813265.S2CID917306.
External links
edit- hier-20atNeuroNames
- Stained brain slice images which include the "cerebral cortex"at theBrainMaps project
- "The primary visual cortex",Webvision: Comprehensive article about the structure and function of the primary visual cortex.
- "Basic cell types",Webvision: Image of the basic cell types of the monkey cerebral cortex.
- Cerebral Cortex – Cell Centered Database