Sulfonamideis afunctional group(a part of amolecule) that is the basis of several groups ofdrugs,which are calledsulphonamides,sulfa drugsorsulpha drugs.The original antibacterial sulfonamides are synthetic (nonantibiotic)antimicrobialagents that contain thesulfonamidegroup. Some sulfonamides are also devoid of antibacterial activity, e.g., theanticonvulsantsultiame.Thesulfonylureasandthiazide diureticsare newer drug groups based upon the antibacterial sulfonamides.[1][2]
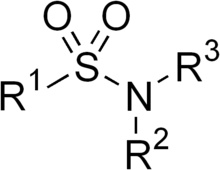



Allergiesto sulfonamides are common. The overall incidence ofadverse drug reactionsto sulfa antibiotics is approximately 3%, close topenicillin;[3]hence medications containing sulfonamides are prescribed carefully.
Sulfonamide drugs were the first broadly effective antibacterials to be used systemically, and paved the way for the antibiotic revolution in medicine.
Function
editIn bacteria, antibacterial sulfonamides act ascompetitive inhibitorsof the enzymedihydropteroate synthase(DHPS), an enzyme involved infolate synthesis.Sulfonamides are therefore bacteriostatic and inhibit growth and multiplication of bacteria, but do not kill them. Humans, in contrast to bacteria, acquirefolate(vitamin B9) through the diet.[4]
Sulfonamides are used to treat allergies and coughs, as well as having antifungal and antimalarial functions. The moiety is also present in other medications that are not antimicrobials, includingthiazidediuretics(includinghydrochlorothiazide,metolazone,andindapamide,among others), loop diuretics (includingfurosemide,bumetanide,andtorsemide),acetazolamide,sulfonylureas(includingglipizide,glyburide,among others), and someCOX-2 inhibitors(e.g.,celecoxib).
Sulfasalazine,in addition to its use as an antibiotic, is also used in the treatment ofinflammatory bowel disease.[5]
History
editSulfonamide drugs were the first broadly effective antibacterials to be used systemically, and paved the way for the antibiotic revolution in medicine. The first sulfonamide, trade-namedProntosil,was aprodrug.Experiments with Prontosil began in 1932 in the laboratories ofBayerAG, at that time a component of the huge German chemical trustIG Farben.The Bayer team believed thatcoal-tar dyeswhich are able to bind preferentially to bacteria and parasites might be used to attack harmful organisms in the body. After years of fruitless trial-and-error work on hundreds of dyes, a team led by physician/researcherGerhard Domagk[6](working under the general direction of IG Farben executiveHeinrich Hörlein) finally found one that worked: a red dye synthesized by Bayer chemistJosef Klarerthat had remarkable effects on stopping some bacterial infections in mice.[7]The first official communication about the breakthrough discovery was not published until 1935, more than two years after the drug was patented by Klarer and his research partner Fritz Mietzsch.[citation needed]
Prontosil, as Bayer named the new drug, was the first medicine ever discovered that could effectively treat a range of bacterial infections inside the body. It had a strong protective action against infections caused bystreptococci,including blood infections,childbed fever,anderysipelas,and a lesser effect on infections caused by other cocci. However, it had no effect at all in the test tube, exerting its antibacterial action only in live animals. Later, it was discovered byDaniel Bovet,[8]Federico Nitti, andJacquesandThérèse Tréfouël,a French research team led byErnest Fourneauat thePasteur Institute,that the drug was metabolized into two parts inside the body, releasing from the inactive dye portion a smaller, colorless, active compound calledsulfanilamide.[9]The discovery helped establish the concept of "bioactivation" and dashed the German corporation's dreams of enormous profit; the active molecule sulfanilamide (or sulfa) had first been synthesized in 1906 and was widely used in the dye-making industry; its patent had since expired and the drug was available to anyone.[10]
The result was a sulfa craze.[11]For several years in the late 1930s, hundreds of manufacturers produced myriad forms of sulfa. This and the lack of testing requirements led to theelixir sulfanilamidedisaster in the fall of 1937, during which at least 100 people were poisoned withdiethylene glycol.This led to the passage of theFederal Food, Drug, and Cosmetic Actin 1938 in the United States. As the first and only effective broad-spectrum antibiotic available in the years beforepenicillin,heavy use of sulfa drugs continued into the early years ofWorld War II.[12]They are credited with saving the lives of tens of thousands of patients, includingFranklin Delano Roosevelt Jr.(son of US PresidentFranklin Delano Roosevelt) andWinston Churchill.[13][14]Sulfa had a central role in preventing wound infections during the war. American soldiers were issued afirst-aid kitcontaining sulfa pills and powder and were told to sprinkle it on any open wound.[15]
The sulfanilamide compound is more active in theprotonatedform. The drug has very low solubility and sometimes can crystallize in the kidneys, due to its first pKaof around 10.[clarification needed]This is a very painful experience, so patients are told to take the medication with copious amounts of water. Newer analogous compounds prevent this complication because they have a lower pKa,around 5–6,[citation needed]making them more likely to remain in a soluble form.
Many thousands of molecules containing the sulfanilamide structure have been created since its discovery (by one account, over 5,400 permutations by 1945), yielding improved formulations with greater effectiveness and less toxicity. Sulfa drugs are still widely used for conditions such as acne and urinary tract infections, and are receiving renewed interest for the treatment of infections caused by bacteria resistant to other antibiotics.[citation needed]
Preparation
editSulfonamides are prepared by the reaction of asulfonyl chloridewith ammonia or an amine. Certain sulfonamides (sulfadiazine orsulfamethoxazole) are sometimes mixed with the drugtrimethoprim,which acts againstdihydrofolate reductase.As of 2013, theRepublic of Irelandis the largest exporter worldwide of sulfonamides, accounting for approximately 32% of total exports.[16]
Varieties
editSide effects
editSulfonamides have the potential to cause a variety ofadverse effects,including urinary tract disorders,haemopoieticdisorders,porphyriaand hypersensitivity reactions. When used in large doses, they may cause a strong allergic reaction. The most serious of these are classified assevere cutaneous adverse reactions(i.e. SCARs) and include theStevens–Johnson syndrome,toxic epidermal necrolysis(also known as Lyell syndrome), theDRESS syndrome,and a not quite as serious SCARs reaction,acute generalized exanthematous pustulosis.Any one of these SCARs may be triggered by certain sulfonamides.[3]
Approximately 3% of the general population have adverse reactions when treated with sulfonamide antimicrobials. Of note is the observation that patients with HIV have a much higher prevalence, at about 60%.[17]
Hypersensitivity reactions are less common in nonantibiotic sulfonamides, and, though controversial, the available evidence suggests those with hypersensitivity to sulfonamide antibiotics do not have an increased risk of hypersensitivity reaction to the nonantibiotic agents.[18]A key component to the allergic response to sulfonamide antibiotics is the arylamine group at N4, found in sulfamethoxazole, sulfasalazine, sulfadiazine, and the anti-retrovirals amprenavir and fosamprenavir. Other sulfonamide drugs do not contain this arylamine group; available evidence suggests that patients who are allergic to arylamine sulfonamides do not cross-react to sulfonamides that lack the arylamine group, and may therefore safely take non-arylamine sulfonamides.[19]It has therefore been argued that the terms "sulfonamide allergy" or "sulfa allergy" are misleading and should be replaced by a reference to a specific drug (e.g., "cotrimoxazoleallergy ").[20]
Two regions of the sulfonamide antibiotic chemical structure are implicated in the hypersensitivity reactions associated with the class.
- The first is the N1 heterocyclic ring, which causes a type Ihypersensitivityreaction.
- The second is the N4 amino nitrogen that, in a stereospecific process, forms reactive metabolites that cause either direct cytotoxicity or immunologic response.
The nonantibiotic sulfonamides lack both of these structures.[21]
The most common manifestations of a hypersensitivity reaction to sulfa drugs arerashandhives.However, there are several life-threatening manifestations of hypersensitivity to sulfa drugs, includingStevens–Johnson syndrome,toxic epidermal necrolysis,agranulocytosis,hemolytic anemia,thrombocytopenia,fulminant hepatic necrosis, andacute pancreatitis,among others.[22]
See also
edit- Dihydropteroate synthase
- Elixir sulfanilamide
- Hellmuth Kleinsorge(1920–2001) German medical doctor
- PABA
- Timeline of antibiotics
References
edit- ^Henry RJ (1943)."The Mode of Action of Sulfonamides".Bacteriological Reviews.7(4): 175–262.doi:10.1128/MMBR.7.4.175-262.1943.PMC440870.PMID16350088.
- ^"SULFONAMIDE CLASS ANTIBIOTICS".chemicalland21.com.Retrieved17 January2014.
- ^ab"Sulfa Drugs Allergy -- Sulfa Bactrim Drug Allergies".allergies.about.com.Retrieved17 January2014.
- ^M. Madigan, J. Martinko, D. Stahl, D. Clark,Brock Biology of Microorganisms (13th ed.),Pearson Education, 2012, p. 797ISBN9780321735515
- ^Lackie, John (2010).A Dictionary of Biomedicine.Oxford University Press. p. 543.ISBN978-0199549351.
- ^Otten H (1986)."Domagk and the development of the sulphonamides".Journal of Antimicrobial Chemotherapy.17(6): 689–696.doi:10.1093/jac/17.6.689.PMID3525495.
- ^Hager, Thomas (1 September 2006).The Demon Under the Microscope: From Battlefield Hospitals to Nazi Labs, One Doctor's Heroic Search for the World's First Miracle Drug.ISBN978-0-307-35228-6.
- ^Cf. Daniel Bovet,Une chimie qui guérit: Histoire de la découverte des sulfamides,Paris, Payot, coll. « Médecine et sociétés », 1988 (ISBN2-228-88108-2).
- ^Tréfouël, J.; Tréfouël, Th.; Nitti, F.; Bovet, D. (23 November 1935). "Activité du p. aminophénylsulfamide sur l'infection streptococcique expérimentale de la souris et du lapin".C. R. Soc. Biol.120:756.
- ^"History of medicine".Encyclopædia Britannica.Retrieved17 January2014.
- ^"Bad Health—Elixir Sulfanilamide".The Blog of Bad.9 February 2009.Retrieved17 January2014.
- ^"History of WWII Medicine".Archived fromthe originalon 14 October 1999.Retrieved4 April2014.
- ^"Medicine: Prontosil".Time.28 December 1936.Retrieved28 March2014.
- ^Kadenczki, Lajos; Szopkó, Henrietta Stefánné (2012)."Adaptation and extension of sulfonamide and other antibiotics determination by solid-phase extraction followed by liquid chromatography and mass spectrometry"(PDF).Geosciences and Engineering.1(1): 147.ISSN2063-6997.OCLC1066656753.
- ^Medical Innovations: AntibioticsThe National WWII Museum. Accessed 29 July 2021.
- ^"Trade of Sulfonamides".Massachusetts Institute of Technology.Retrieved26 October2013.
- ^Tilles SA (August 2001). "Practical issues in the management of hypersensitivity reactions: sulfonamides".Southern Medical Journal.94(8): 817–24.doi:10.1097/00007611-200108000-00013.PMID11549195.S2CID8493824.
- ^Slatore CG, Tilles SA (2004). "Sulfonamide hypersensitivity".Immunology and Allergy Clinics of North America.24(3): 477–490, vii.doi:10.1016/j.iac.2004.03.011.PMID15242722.
- ^Knowles S, Shapiro L, Shear NH (2001). "Should Celecoxib Be Contraindicated in Patients Who Are Allergic to Sulfonamides?".Drug Safety.24(4): 239–247.doi:10.2165/00002018-200124040-00001.PMID11330653.S2CID20386434.
- ^Veroni M."ALLERGIES TO SULFONAMIDE ANTIBIOTICS AND CROSS-REACTIVITIES"(PDF).Western Australian Therapeutic Advisory Group. Archived fromthe original(PDF)on 3 March 2011.Retrieved7 February2014.
- ^Brackett CC, Singh H, Block JH (July 2004). "Likelihood and mechanisms of cross-allergenicity between sulfonamide antibiotics and other drugs containing a sulfonamide functional group".Pharmacotherapy.24(7): 856–70.doi:10.1592/phco.24.9.856.36106.PMID15303450.S2CID25623592.
- ^Harrison's Principles of Internal Medicine, 13th Ed.McGraw-Hill Inc. 1994. p. 604.