Cell surface receptors(membrane receptors,transmembrane receptors) arereceptorsthat are embedded in theplasma membraneofcells.[1]They act incell signalingby receiving (binding to)extracellular molecules.They are specializedintegral membrane proteinsthat allow communication between the cell and theextracellular space.The extracellular molecules may behormones,neurotransmitters,cytokines,growth factors,cell adhesion molecules,ornutrients;they react with the receptor to induce changes in themetabolismand activity of a cell. In the process ofsignal transduction,ligand bindingaffects acascading chemical changethrough the cell membrane.
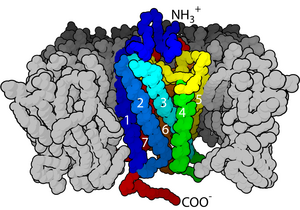
Structure and mechanism
editMany membrane receptors aretransmembrane proteins.There are various kinds, includingglycoproteinsandlipoproteins.[2]Hundreds of different receptors are known and many more have yet to be studied.[3][4]Transmembrane receptors are typically classified based on theirtertiary(three-dimensional) structure. If the three-dimensional structure is unknown, they can be classified based onmembrane topology.In the simplest receptors,polypeptide chainscross thelipid bilayeronce, while others, such as theG-protein coupled receptors,cross as many as seven times. Eachcell membranecan have several kinds of membrane receptors, with varying surface distributions. A single receptor may also be differently distributed at different membrane positions, depending on the sort of membrane and cellular function. Receptors are often clustered on the membrane surface, rather than evenly distributed.[5][6]
Mechanism
editTwo models have been proposed to explain transmembrane receptors' mechanism of action.
- Dimerization:The dimerization model suggests that prior to ligand binding, receptors exist in amonomericform. When agonist binding occurs, the monomers combine to form an activedimer.
- Rotation:Ligand binding to the extracellular part of the receptor induces a rotation (conformational change) of part of the receptor's transmembrane helices. The rotation alters which parts of the receptor are exposed on the intracellular side of the membrane, altering how the receptor can interact with other proteins within the cell.[7]
Domains
editP=plasma membrane
I=intracellular space
Transmembrane receptors inplasma membranecan usually be divided into three parts.
Extracellular domains
editThe extracellular domain is just externally from the cell ororganelle.If the polypeptide chain crosses the bilayer several times, the external domain comprises loops entwined through the membrane. By definition, a receptor's main function is to recognize and respond to a type of ligand. For example, aneurotransmitter,hormone,or atomic ions may each bind to the extracellular domain as a ligand coupled to receptor.Klothois an enzyme which effects a receptor to recognize the ligand (FGF23).
Transmembrane domains
editTwo most abundant classes of transmembrane receptors areGPCRandsingle-pass transmembrane proteins.[8][9]In some receptors, such as thenicotinic acetylcholine receptor,the transmembrane domain forms a protein pore through the membrane, or around theion channel.Upon activation of an extracellular domain by binding of the appropriate ligand, the pore becomes accessible to ions, which then diffuse. In other receptors, the transmembrane domains undergo a conformational change upon binding, which affects intracellular conditions. In some receptors, such as members of the7TM superfamily,the transmembrane domain includes a ligand binding pocket.
Intracellular domains
editThe intracellular (orcytoplasmic) domain of the receptor interacts with the interior of the cell or organelle, relaying the signal. There are two fundamental paths for this interaction:
- The intracellular domain communicates via protein-protein interactions againsteffector proteins,which in turn pass a signal to the destination.
- Withenzyme-linked receptors,the intracellular domain hasenzymatic activity.Often, this istyrosine kinaseactivity. The enzymatic activity can also be due to an enzyme associated with the intracellular domain.
Signal transduction
editSignal transductionprocesses through membrane receptors involve the external reactions, in which the ligand binds to a membrane receptor, and the internal reactions, in which intracellular response is triggered.[10][11]
Signal transduction through membrane receptors requires four parts:
- Extracellular signaling molecule: an extracellular signaling molecule is produced by one cell and is at least capable of traveling to neighboring cells.
- Receptor protein: cells must have cell surface receptor proteins which bind to the signaling molecule and communicate inward into the cell.
- Intracellular signaling proteins: these pass the signal to the organelles of the cell. Binding of the signal molecule to the receptor protein will activate intracellular signaling proteins that initiate a signaling cascade.
- Target proteins: the conformations or other properties of the target proteins are altered when a signaling pathway is active and changes the behavior of the cell.[11]
Membrane receptors are mainly divided by structure and function into 3 classes: Theion channel linked receptor;Theenzyme-linked receptor;and TheG protein-coupled receptor.
- Ion channel linked receptorshave ion channels for anions and cations, and constitute a large family of multipass transmembrane proteins. They participate in rapid signaling events usually found in electrically active cells such asneurons.They are also calledligand-gated ion channels.Opening and closing of ion channels is controlled byneurotransmitters.
- Enzyme-linked receptorsare either enzymes themselves, or directly activate associated enzymes. These are typically single-pass transmembrane receptors, with the enzymatic component of the receptor kept intracellular. The majority of enzyme-linked receptors are, or associate with, protein kinases.
- G protein-coupled receptorsare integral membrane proteins that possess seven transmembrane helices. These receptors activate aG proteinuponagonistbinding, and the G-protein mediates receptor effects on intracellular signaling pathways.
Ion channel-linked receptor
editDuring the signal transduction event in a neuron, the neurotransmitter binds to the receptor and alters the conformation of the protein. This opens the ion channel, allowing extracellular ions into the cell. Ion permeability of the plasma membrane is altered, and this transforms the extracellular chemical signal into an intracellular electric signal which alters thecell excitability.[12]
Theacetylcholine receptoris a receptor linked to a cation channel. The protein consists of four subunits: alpha (α), beta (β), gamma (γ), and delta (δ) subunits. There are two α subunits, with oneacetylcholinebinding site each. This receptor can exist in three conformations. The closed and unoccupied state is the native protein conformation. As two molecules of acetylcholine both bind to the binding sites on α subunits, the conformation of the receptor is altered and the gate is opened, allowing for the entry of many ions and small molecules. However, this open and occupied state only lasts for a minor duration and then the gate is closed, becoming the closed and occupied state. The two molecules of acetylcholine will soon dissociate from the receptor, returning it to the native closed and unoccupied state.[13][14]
Enzyme-linked receptors
editAs of 2009, there are 6 known types ofenzyme-linked receptors:Receptortyrosine kinases;Tyrosine kinase associated receptors; Receptor-liketyrosine phosphatases;Receptorserine/threoninekinases;Receptorguanylyl cyclasesandhistidine kinaseassociated receptors. Receptor tyrosine kinases have the largest population and widest application. The majority of these molecules are receptors forgrowth factorssuch asepidermal growth factor(EGF),platelet-derived growth factor(PDGF),fibroblast growth factor(FGF),hepatocyte growth factor(HGF),nerve growth factor(NGF) andhormonessuch asinsulin. Most of these receptors will dimerize after binding with their ligands, in order to activate further signal transductions. For example, after theepidermal growth factor (EGF)receptor binds with its ligand EGF, the two receptors dimerize and then undergophosphorylationof thetyrosineresidues in the enzyme portion of each receptor molecule. This will activate the tyrosine kinase and catalyze further intracellular reactions.
G protein-coupled receptors
editG protein-coupled receptors comprise a largeproteinfamily of transmembrane receptors. They are found only ineukaryotes.[15]Theligandswhich bind and activate these receptors include: photosensitive compounds,odors,pheromones,hormones,andneurotransmitters.These vary in size from small molecules topeptidesand largeproteins.G protein-coupled receptors are involved in many diseases, and thus are the targets of many modern medicinal drugs.[16]
There are two principal signal transduction pathways involving the G-protein coupled receptors: thecAMPsignaling pathway and thephosphatidylinositolsignaling pathway.[17]Both are mediated viaG proteinactivation. The G-protein is a trimeric protein, with three subunits designated as α, β, and γ. In response to receptor activation, the α subunit releases boundguanosine diphosphate(GDP), which is displaced byguanosine triphosphate(GTP), thus activating the α subunit, which then dissociates from the β and γ subunits. The activated α subunit can further affect intracellular signaling proteins or target functional proteins directly.
Membrane receptor-related disease
editIf the membrane receptors are denatured or deficient, the signal transduction can be hindered and cause diseases. Some diseases are caused by disorders of membrane receptor function. This is due to deficiency or degradation of the receptor via changes in the genes that encode and regulate the receptor protein. The membrane receptorTM4SF5influences the migration of hepatic cells andhepatoma.[18]Also, the cortical NMDA receptor influences membrane fluidity, and is altered in Alzheimer's disease.[19]When the cell is infected by a non-enveloped virus, the virus first binds to specific membrane receptors and then passes itself or a subviral component to the cytoplasmic side of the cellular membrane. In the case ofpoliovirus,it is known in vitro that interactions with receptors cause conformational rearrangements which release a virion protein called VP4.The N terminus of VP4 is myristylated and thus hydrophobic【myristic acid=CH3(CH2)12COOH】. It is proposed that the conformational changes induced by receptor binding result in the attachment of myristic acid on VP4 and the formation of a channel for RNA.
Structure-based drug design
editThrough methods such asX-ray crystallographyandNMR spectroscopy,the information about 3D structures of target molecules has increased dramatically, and so has structural information about the ligands. This drives rapid development ofstructure-based drug design.Some of these new drugs target membrane receptors. Current approaches to structure-based drug design can be divided into two categories. The first category is about determining ligands for a given receptor. This is usually accomplished through database queries, biophysical simulations, and the construction of chemical libraries. In each case, a large number of potential ligand molecules are screened to find those fitting the binding pocket of the receptor. This approach is usually referred to as ligand-based drug design. The key advantage of searching a database is that it saves time and power to obtain new effective compounds. Another approach of structure-based drug design is about combinatorially mapping ligands, which is referred to as receptor-based drug design. In this case, ligand molecules are engineered within the constraints of a binding pocket by assembling small pieces in a stepwise manner. These pieces can be either atoms or molecules. The key advantage of such a method is that novel structures can be discovered.[20][21][22]
Other examples
editSee also
editReferences
edit- ^"9.3: Signaling Molecules and Cellular Receptors - Types of Receptors".Biology LibreTexts.12 July 2018.Retrieved24 July2023.
- ^Cuatrecasas P. (1974). "Membrane Receptors".Annual Review of Biochemistry.43:169–214.doi:10.1146/annurev.bi.43.070174.001125.PMID4368906.S2CID44727052.
- ^Dautzenberg FM, Hauger RL (February 2002). "The CRF peptide family and their receptors: yet more partners discovered".Trends Pharmacol. Sci.23(2): 71–7.doi:10.1016/S0165-6147(02)01946-6.PMID11830263.
- ^Rivière S, Challet L, Fluegge D, Spehr M, Rodriguez I (May 2009). "Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors".Nature.459(7246): 574–7.doi:10.1038/nature08029.PMID19387439.
- ^Rothberg K.G.; Ying Y.S.; Kamen B.A.; Anderson R.G. (1990)."Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate".The Journal of Cell Biology.111(6): 2931–2938.doi:10.1083/jcb.111.6.2931.PMC2116385.PMID2148564.
- ^Jacobson C.; Côté P.D.; Rossi S.G.; Rotundo R.L.; Carbonetto S. (2001)."The Dystroglycan Complex Is Necessary for Stabilization of Acetylcholine Receptor Clusters at Neuromuscular Junctions and Formation of the Synaptic Basement Membrane".The Journal of Cell Biology.152(3): 435–450.doi:10.1083/jcb.152.3.435.PMC2195998.PMID11157973.
- ^Maruyama, Ichiro N. (2015-09-01)."Activation of transmembrane cell-surface receptors via a common mechanism? The" rotation model "".BioEssays.37(9): 959–967.doi:10.1002/bies.201500041.ISSN1521-1878.PMC5054922.PMID26241732.
- ^Superfamilies of single-pass transmembrane receptorsinMembranome database
- ^Superfamilies of single-pass transmembrane protein ligands and regulators of receptorsinMembranome database
- ^Ullricha A., Schlessingerb J.; Schlessinger, J (1990). "Signal transduction by receptors with tyrosine kinase activity".Cell.61(2): 203–212.doi:10.1016/0092-8674(90)90801-K.PMID2158859.
- ^abKenneth B. Storey (1990).Functional Metabolism.Wiley-IEEE. pp. 87–94.ISBN978-0-471-41090-4.
- ^Hille B. (2001).Ion channels of excitable membranes.Sunderland, Mass.ISBN978-0-87893-321-1.
- ^Miyazawa A.; Fujiyoshi Y.; Unwin N. (2003). "Structure and gating mechanism of the acetylcholine receptor pore".Nature.423(6943): 949–955.doi:10.1038/nature01748.PMID12827192.
- ^Akabas M.H.; Stauffer D.A.; Xu M.; Karlin A. (1992). "Acetylcholine receptor channel structure probed in cysteine-substitution mutants".Science.258(5080): 307–310.doi:10.1126/science.1384130.PMID1384130.
- ^King N, Hittinger CT, Carroll SB (2003). "Evolution of key cell signaling and adhesion protein families predates animal origins".Science.301(5631): 361–3.doi:10.1126/science.1083853.PMID12869759.
- ^Filmore, David (2004)."It's a GPCR world".Modern Drug Discovery.2004(November): 24–28.
- ^Gilman A.G. (1987). "G Proteins: Transducers of Receptor-Generated Signals".Annual Review of Biochemistry.56:615–649.doi:10.1146/annurev.bi.56.070187.003151.PMID3113327.S2CID33992382.
- ^Müller-Pillascha F.; Wallrappa C.; Lachera U.; Friessb H.; Büchlerb M.; Adlera G.; Gress T. M. (1998). "Identification of a new tumour-associated antigen TM4SF5 and its expression in human cancer".Gene.208(1): 25–30.doi:10.1016/S0378-1119(97)00633-1.PMID9479038.
- ^Scheuer K.; Marasb A.; Gattazb W.F.; Cairnsc N.; Förstlb H.; Müller W.E. (1996). "Cortical NMDA Receptor Properties and Membrane Fluidity Are Altered in Alzheimer's Disease".Dementia.7(4): 210–214.doi:10.1159/000106881.PMID8835885.
- ^Wang R.; Gao Y.; Lai L. (2000). "LigBuilder: A Multi-Purpose Program for Structure-Based Drug Design".Journal of Molecular Modeling.6(7–8): 498–516.doi:10.1007/s0089400060498.
- ^Schneider G.; Fechner U. (2005). "Computer-based de novo design of drug-like molecules".Nature Reviews Drug Discovery.4(8): 649–663.doi:10.1038/nrd1799.PMID16056391.
- ^Jorgensen W.L. (2004). "The Many Roles of Computation in Drug Discovery".Science.303(5665): 1813–1818.doi:10.1126/science.1096361.PMID15031495.S2CID1307935.
External links
edit- IUPHAR GPCR DatabaseArchived2019-03-23 at theWayback Machine
- Cell+Surface+Receptorsat the U.S. National Library of MedicineMedical Subject Headings(MeSH)