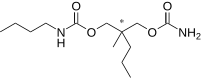
Tybamate(INN;Solacen,Tybatran,Effisax) is ananxiolyticof thecarbamatefamily.[1]It is aprodrugformeprobamatein the same way as the better known drugcarisoprodol.It has liver enzyme inducing effects similar to those ofphenobarbitalbut much weaker.[2]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.022.050 |
| Chemical and physical data | |
| Formula | C13H26N2O4 |
| Molar mass | 274.361g·mol−1 |
| 3D model (JSmol) | |
| |
| |
As the trade name Tybatran (Robins), it was formerly available in capsules of 125, 250, and 350 mg, taken 3 or 4 times a day for a total daily dosage of 750 mg to 2 g. The plasma half-life of the drug is three hours. At high doses in combination withphenothiazines,it could produce convulsions.[3]
Synthesis
editCatalytic hydrogenationof 2-methyl-2-pentenal (1) gives thealdehyde2-methylpentanal (2). Treatment withformaldehydegives acrossed Cannizzaro reactionyielding2,2-bis(hydroxymethyl)pentane(3).Cyclisationof thisdiolwithdiethyl carbonategives (4), which reacts with ammonia to provide thecarbamate(5). Lastly, treatment with butyl isocyanate (6) produces tybamate.[4][5][6]
References
edit- ^Index Nominum 2000: International Drug Directory.Taylor & Francis. January 2000. p. 1077.ISBN978-3-88763-075-1.
- ^Segelman FH, Kelton E, Terzi RM, Kucharczyk N, Sofia RD (June 1985). "The comparative potency of phenobarbital and five 1,3-propanediol dicarbamates for hepatic cytochrome P450 induction in rats".Research Communications in Chemical Pathology and Pharmacology.48(3): 467–70.PMID4023427.
- ^American Medical Association Dept of Drugs (1977).AMA Drug Evaluations(3rd ed.). Littleton, Mass.: Pub. Sciences Group. p. 406.ISBN978-0-88416-175-2.OCLC1024170745.
- ^Ludwig BJ, Piech EC (1951). "Some Anticonvulsant Agents Derived from 1,3-Propanediols".Journal of the American Chemical Society.73(12): 5779–5781.doi:10.1021/ja01156a086.
- ^Reisberg P, Kress J, Bodin JI (1975).Tybamate.Analytical Profiles of Drug Substances. Vol. 4. pp. 494–515.doi:10.1016/S0099-5428(08)60025-8.ISBN978-0-12-260804-9.
- ^"Tybamate".Pharmaceutical Substances.Thieme.Retrieved2024-07-08.