Avaccineis a biologicalpreparationthat provides activeacquired immunityto a particularinfectiousormalignantdisease.[1][2]The safety and effectiveness of vaccines has been widely studied and verified.[3][4]A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins. The agent stimulates the body's immune system to recognize the agent as a threat, destroy it, and recognize further and destroy any of the microorganisms associated with that agent that it may encounter in the future.
| Vaccine | |
|---|---|
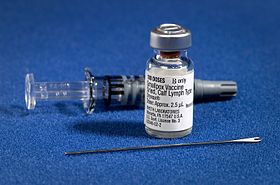 Smallpox vaccineand equipment for administering it | |
| MeSH | D014612 |
Vaccines can beprophylactic(to prevent or alleviate the effects of a futureinfectionby a natural or "wild"pathogen), ortherapeutic(to fight a disease that has already occurred, such ascancer).[5][6][7][8]Some vaccines offer fullsterilizing immunity,in which infection is prevented.[9]
The administration of vaccines is calledvaccination.Vaccination is the most effective method of preventing infectious diseases;[10]widespread immunity due to vaccination is largely responsible for theworldwide eradicationofsmallpoxand the restriction of diseases such aspolio,measles,andtetanusfrom much of the world. TheWorld Health Organization(WHO) reports that licensed vaccines are currently available for twenty-five differentpreventable infections.[11]
The first recorded use ofinoculationto prevent smallpox occurred in the 16th century in China, with the earliest hints of the practice in China coming during the 10th century.[12]It was also the first disease for which a vaccine was produced.[13][14]The folk practice ofinoculationagainstsmallpoxwas brought fromTurkeyto Britain in 1721 byLady Mary Wortley Montagu.[15] The termsvaccineandvaccinationare derived fromVariolae vaccinae(smallpox of the cow), the term devised byEdward Jenner(who both developed the concept of vaccines and created the first vaccine) to denotecowpox.He used the phrase in 1798 for the long title of hisInquiry into the Variolae vaccinae Known as the Cow Pox,in which he described the protective effect of cowpox against smallpox.[16]In 1881, to honor Jenner,Louis Pasteurproposed that the terms should be extended to cover the new protective inoculations then being developed.[17]The science of vaccine development and production is termedvaccinology.

Effects
There is overwhelming scientific consensus that vaccines are a very safe and effective way to fight and eradicate infectious diseases.[19][20][21][22]Theimmune systemrecognizes vaccine agents as foreign, destroys them, and "remembers" them. When thevirulentversion of an agent is encountered, the body recognizes the protein coat on the agent, and thus is prepared to respond, by first neutralizing the target agent before it can enter cells, and secondly by recognizing and destroying infected cells before that agent can multiply to vast numbers.[23][24]
Limitations to their effectiveness, nevertheless, exist.[25]Sometimes, protection fails for vaccine-related reasons such as failures in vaccine attenuation, vaccination regimens or administration.[26]
Failure may also occur for host-related reasons if the host's immune system does not respond adequately or at all. Host-related lack of response occurs in an estimated 2-10% of individuals, due to factors including genetics, immune status, age, health and nutritional status.[26]One type ofprimary immunodeficiencydisorder resulting in genetic failure isX-linked agammaglobulinemia,in which the absence of an enzyme essential forB celldevelopment prevents the host's immune system from generatingantibodiesto apathogen.[27][28]
Host–pathogen interactions and responses to infection are dynamic processes involving multiple pathways in the immune system.[29][30]A host does not develop antibodies instantaneously: while the body'sinnate immunitymay be activated in as little as twelve hours,adaptive immunitycan take 1–2 weeks to fully develop. During that time, the host can still become infected.[31]
Once antibodies are produced, they may promote immunity in any of several ways, depending on the class of antibodies involved. Their success in clearing or inactivating a pathogen will depend on the amount of antibodies produced and on the extent to which those antibodies are effective at countering the strain of the pathogen involved, since different strains may be differently susceptible to a given immune reaction.[30] In some cases vaccines may result in partial immune protection (in which immunity is less than 100% effective but still reduces risk of infection) or in temporary immune protection (in which immunity wanes over time) rather than full or permanent immunity. They can still raise the reinfection threshold for the population as a whole and make a substantial impact.[32]They can also mitigate the severity of infection, resulting in a lowermortality rate,lowermorbidity,faster recovery from illness, and a wide range of other effects.[33][34]
Those who are older often display less of a response than those who are younger, a pattern known asImmunosenescence.[35] Adjuvantscommonly are used to boost immune response, particularly for older people whose immune response to a simple vaccine may have weakened.[36]
Theefficacyor performance of the vaccine is dependent on several factors:
- the disease itself (for some diseases vaccination performs better than for others)
- the strain of vaccine (some vaccines are specific to, or at least most effective against, particular strains of the disease)[37]
- whether thevaccination schedulehas been properly observed.
- idiosyncratic response to vaccination; some individuals are "non-responders" to certain vaccines, meaning that they do not generate antibodies even after being vaccinated correctly.
- assorted factors such as ethnicity, age, or genetic predisposition.
If a vaccinated individual does develop the disease vaccinated against (breakthrough infection), the disease is likely to be less virulent than in unvaccinated cases.[38]
Important considerations in an effective vaccination program:[39]
- careful modeling to anticipate the effect that an immunization campaign will have on the epidemiology of the disease in the medium to long term
- ongoing surveillance for the relevant disease following introduction of a new vaccine
- maintenance of high immunization rates, even when a disease has become rare
In 1958, there were 763,094 cases of measles in the United States; 552 deaths resulted.[40][41]After the introduction of new vaccines, the number of cases dropped to fewer than 150 per year (median of 56).[41]In early 2008, there were 64 suspected cases of measles. Fifty-four of those infections were associated with importation from another country, although only thirteen percent were actually acquired outside the United States; 63 of the 64 individuals either had never been vaccinated against measles or were uncertain whether they had been vaccinated.[41]
Vaccines led to the eradication ofsmallpox,one of the most contagious and deadly diseases in humans.[42]Other diseases such as rubella,polio,measles, mumps,chickenpox,andtyphoidare nowhere near as common as they were a hundred years ago thanks to widespread vaccination programs. As long as the vast majority of people are vaccinated, it is much more difficult for an outbreak of disease to occur, let alone spread. This effect is calledherd immunity.Polio, which is transmitted only among humans, is targeted by an extensiveeradication campaignthat has seen endemic polio restricted to only parts of three countries (Afghanistan, Nigeria, and Pakistan).[43]However, the difficulty of reaching all children, cultural misunderstandings, anddisinformationhave caused the anticipated eradication date to be missed several times.[44][45][46][47]
Vaccines also help prevent the development of antibiotic resistance. For example, by greatly reducing the incidence of pneumonia caused byStreptococcus pneumoniae,vaccine programs have greatly reduced the prevalence of infections resistant to penicillin or other first-line antibiotics.[48]
The measles vaccine is estimated to prevent a million deaths every year.[49]
Adverse effects
Vaccinations given to children, adolescents, or adults are generally safe.[50][51]Adverse effects, if any, are generally mild.[52]The rate of side effects depends on the vaccine in question.[52]Some common side effects include fever, pain around the injection site, and muscle aches.[52]Additionally, some individuals may be allergic to ingredients in the vaccine.[53]MMR vaccineis rarely associated withfebrile seizures.[51]
Host-( "vaccinee" )-related determinants that render a person susceptible to infection, such asgenetics,health status (underlying disease, nutrition, pregnancy,sensitivitiesorallergies),immune competence,age, andeconomic impactorcultural environmentcan be primary or secondary factors affecting the severity of infection and response to a vaccine.[26]Elderly (above age 60),allergen-hypersensitive,andobesepeople have susceptibility to compromisedimmunogenicity,which prevents or inhibits vaccine effectiveness, possibly requiring separate vaccine technologies for these specific populations or repetitivebooster vaccinationsto limitvirus transmission.[26]
Severe side effects are extremely rare.[51]Varicella vaccineis rarely associated with complications inimmunodeficientindividuals, androtavirus vaccinesare moderately associated withintussusception.[51]
At least 19 countries have no-fault compensation programs to provide compensation for those with severe adverse effects of vaccination.[54]The United States' program is known as theNational Childhood Vaccine Injury Act,and the United Kingdom employs theVaccine Damage Payment.
Types
Vaccines typically contain attenuated, inactivated or dead organisms or purified products derived from them. There are several types of vaccines in use.[55]These represent different strategies used to try to reduce the risk of illness while retaining the ability to induce a beneficial immune response.
Attenuated
Some vaccines contain live,attenuatedmicroorganisms. Many of these are activevirusesthat have been cultivated under conditions that disable their virulent properties, or that use closely related but less dangerous organisms to produce a broad immune response. Although most attenuated vaccines are viral, some are bacterial in nature. Examples include the viral diseasesyellow fever,measles,mumps,andrubella,and the bacterial diseasetyphoid.The liveMycobacteriumtuberculosisvaccine developed by Calmette and Guérin is not made of acontagiousstrain but contains a virulently modified strain called "BCG"used to elicit an immune response to the vaccine. The live attenuated vaccine containing strainYersinia pestisEV is used for plague immunization. Attenuated vaccines have some advantages and disadvantages. Attenuated, or live, weakened, vaccines typically provoke more durable immunological responses. But they may not be safe for use in immunocompromised individuals, and on rare occasions mutate to a virulent form and cause disease.[56]
Inactivated
Some vaccines contain inactivated, but previously virulent, micro-organisms that have been destroyed with chemicals, heat, or radiation[57]– "ghosts", with intact but empty bacterial cell envelopes. They are considered an intermediate phase between the inactivated and attenuated vaccines.[58]Examples include IPV (polio vaccine),hepatitis A vaccine,rabies vaccineand mostinfluenza vaccines.[59]
Toxoid
Toxoidvaccines are made from inactivated toxic compounds that cause illness rather than the micro-organism.[59]Examples of toxoid-based vaccines includetetanusanddiphtheria.[59]Not all toxoids are for micro-organisms; for example,Crotalus atroxtoxoid is used to vaccinate dogs againstrattlesnakebites.[60]
Subunit
Rather than introducing an inactivated or attenuated micro-organism to an immune system (which would constitute a "whole-agent" vaccine), asubunitvaccine uses a fragment of it to create an immune response. One example is the subunit vaccine againsthepatitisB,which is composed of only the surface proteins of the virus (previously extracted from theblood serumof chronically infected patients but now produced byrecombinationof the viral genes intoyeast).[61]Another example isedible algae vaccines,such as thevirus-like particle(VLP) vaccine againsthuman papillomavirus(HPV), which is composed of the viral majorcapsidprotein.[62]Another example is thehemagglutininandneuraminidasesubunits of theinfluenzavirus.[59]A subunit vaccine is being used for plague immunization.[63]
Conjugate
Certain bacteria have a polysaccharideouter coatthat is poorlyimmunogenic.By linking these outer coats to proteins (e.g., toxins), theimmune systemcan be led to recognize thepolysaccharideas if it were a protein antigen. This approach is used in theHaemophilus influenzaetype B vaccine.[64]
Outer membrane vesicle
Outer membrane vesicles(OMVs) are naturally immunogenic and can be manipulated to produce potent vaccines. The best known OMV vaccines are those developed forserotype B meningococcal disease.[65][66]
Heterotypic
Heterologous vaccinesalso known as "Jennerian vaccines", are vaccines that are pathogens of other animals that either do not cause disease or cause mild disease in the organism being treated. The classic example is Jenner's use of cowpox to protect against smallpox. A current example is the use ofBCG vaccinemade fromMycobacterium bovisto protect againsttuberculosis.[67]
Genetic vaccine
Genetic vaccines are based on the principle of uptake of a nucleic acid into cells, whereupon a protein is produced according to the nucleic acid template. This protein is usually the immunodominant antigen of the pathogen or a surface protein that enables the formation of neutralizing antibodies. The subgroup of genetic vaccines encompass viral vector vaccines, RNA vaccines and DNA vaccines.[citation needed]
Viral vector
Viral vector vaccines use a safevirusto insert pathogen genes in the body to produce specificantigens,such as surfaceproteins,to stimulate animmune response.[68][69]
RNA
An mRNA vaccine (orRNA vaccine) is a novel type of vaccine which is composed of the nucleic acid RNA, packaged within a vector such as lipidnanoparticles.[70]Among theCOVID-19 vaccinesare a number of RNA vaccines to combat theCOVID-19 pandemicand some have been approved or have receivedemergency use authorizationin some countries. For example, thePfizer-BioNTechvaccine andModerna mRNAvaccine are approved for use in adults and children in the US.[71][72][73]
DNA
A DNA vaccine uses aDNAplasmid(pDNA)) that encodes for an antigenic protein originating from the pathogen upon which the vaccine will be targeted. pDNA is inexpensive, stable, and relatively safe, making it an excellent option for vaccine delivery.[74]
This approach offers a number of potential advantages over traditional approaches, including the stimulation of both B- and T-cell responses, improved vaccine stability, the absence of any infectious agent and the relative ease of large-scale manufacture.[75]
Experimental
Many innovative vaccines are also in development and use.
- Dendritic cell vaccines combinedendritic cellswith antigens to present the antigens to the body's white blood cells, thus stimulating an immune reaction. These vaccines have shown some positive preliminary results for treating brain tumors[76]and are also tested in malignant melanoma.[77]
- Recombinantvector– by combining the physiology of one micro-organism and theDNAof another, immunity can be created against diseases that have complex infection processes. An example is theRVSV-ZEBOV vaccinelicensed to Merck that is being used in 2018 to combatebola in Congo.[78]
- T-cell receptorpeptide vaccines are under development for several diseases using models ofValley Fever,stomatitis,andatopic dermatitis.These peptides have been shown to modulatecytokineproduction and improve cell-mediated immunity.
- Targeting of identified bacterial proteins that are involved in complement inhibition would neutralize the key bacterial virulence mechanism.[79]
- The use ofplasmidshas been validated in preclinical studies as a protective vaccine strategy for cancer and infectious diseases. However, in human studies, this approach has failed to provide clinically relevant benefit. The overall efficacy of plasmid DNA immunization depends on increasing the plasmid'simmunogenicitywhile also correcting for factors involved in the specific activation of immune effector cells.[80]
- Bacterial vector– Similar in principle toviral vector vaccines,but using bacteria instead.[65]
- Antigen-presenting cell[65]
- Technologies which may allow rapid vaccine deployment in response to anovel pathogeninclude the use ofvirus-like particles[81]or protein nanoparticles.[82]
While most vaccines are created using inactivated or attenuated compounds from micro-organisms,synthetic vaccinesare composed mainly or wholly of synthetic peptides, carbohydrates, or antigens.[citation needed]
Valence
Vaccines may bemonovalent(also calledunivalent) ormultivalent(also calledpolyvalent). A monovalent vaccine is designed to immunize against a single antigen or single microorganism.[83]A multivalent or polyvalent vaccine is designed to immunize against two or more strains of the same microorganism, or against two or more microorganisms.[84]The valency of a multivalent vaccine may be denoted with a Greek or Latin prefix (e.g.,bivalent,trivalent,ortetravalent/quadrivalent). In certain cases, a monovalent vaccine may be preferable for rapidly developing a strong immune response.[85]
Interactions
When two or more vaccines are mixed in the same formulation, the two vaccines can interfere. This most frequently occurs with live attenuated vaccines, where one of the vaccine components is more robust than the others and suppresses the growth and immune response to the other components.[86]
This phenomenon was first[when?]noted in the trivalent Sabinpolio vaccine,where the amount of serotype2 virus in the vaccine had to be reduced to stop it from interfering with the "take" of the serotype1 and3 viruses in the vaccine.[87]It was also noted in a 2001 study to be a problem withdenguevaccines, where the DEN-3 serotype was found to predominate and suppress the response to DEN-1, -2 and -4 serotypes.[88]
Other contents
Adjuvants
Vaccines typically contain one or moreadjuvants,used to boost the immune response. Tetanus toxoid, for instance, is usually adsorbed ontoalum.This presents the antigen in such a way as to produce a greater action than the simple aqueous tetanus toxoid. People who have an adverse reaction to adsorbed tetanus toxoid may be given the simple vaccine when the time comes for a booster.[89]
In the preparation for the 1990 Persian Gulf campaign, the whole cellpertussisvaccine was used as an adjuvant foranthraxvaccine. This produces a more rapid immune response than giving only the anthrax vaccine, which is of some benefit if exposure might be imminent.[90]
Preservatives
Vaccines may also contain preservatives to prevent contamination withbacteriaorfungi.Until recent years, the preservativethiomersal(a.k.a.Thimerosalin the US and Japan) was used in many vaccines that did not contain live viruses. As of 2005, the only childhood vaccine in the U.S. that contains thiomersal in greater than trace amounts is the influenza vaccine,[91]which is currently recommended only for children with certain risk factors.[92]Single-dose influenza vaccines supplied in the UK do not list thiomersal in the ingredients. Preservatives may be used at various stages of the production of vaccines, and the most sophisticated methods of measurement might detect traces of them in the finished product, as they may in the environment and population as a whole.[93]
Many vaccines need preservatives to prevent serious adverse effects such asStaphylococcusinfection, which in one 1928 incident killed 12 of 21 children inoculated with adiphtheriavaccine that lacked a preservative.[94]Several preservatives are available, including thiomersal,phenoxyethanol,andformaldehyde.Thiomersal is more effective against bacteria, has a better shelf-life, and improves vaccine stability, potency, and safety; but, in the U.S., theEuropean Union,and a few other affluent countries, it is no longer used as a preservative in childhood vaccines, as a precautionary measure due to itsmercurycontent.[95]Althoughcontroversial claimshave been made that thiomersal contributes toautism,no convincing scientific evidence supports these claims.[96]Furthermore, a 10–11-year study of 657,461 children found that the MMR vaccine does not cause autism and actually reduced the risk of autism by seven percent.[97][98]
Excipients
Beside the active vaccine itself, the followingexcipientsand residual manufacturing compounds are present or may be present in vaccine preparations:[99]
- Aluminumsalts or gels are added asadjuvants.Adjuvants are added to promote an earlier, more potent response, and more persistent immune response to the vaccine; they allow for a lower vaccine dosage.
- Antibioticsare added to some vaccines to prevent the growth of bacteria during production and storage of the vaccine.
- Eggproteinis present in theinfluenza vaccineandyellow fever vaccineas they are prepared using chicken eggs. Other proteins may be present.
- Formaldehydeis used to inactivate bacterial products for toxoid vaccines. Formaldehyde is also used to inactivate unwanted viruses and kill bacteria that might contaminate the vaccine during production.
- Monosodium glutamate(MSG) and 2-phenoxyethanolare used as stabilizers in a few vaccines to help the vaccine remain unchanged when the vaccine is exposed to heat, light, acidity, or humidity.
- Thiomersalis a mercury-containing antimicrobial that is added to vials of vaccines that contain more than one dose to prevent contamination and growth of potentially harmful bacteria. Due to the controversy surrounding thiomersal, it has been removed from most vaccines except multi-use influenza, where it was reduced to levels so that a single dose contained less than a microgram of mercury, a level similar to eating ten grams of canned tuna.[100]
Nomenclature
Various fairly standardized abbreviations for vaccine names have developed, although the standardization is by no means centralized or global. For example, the vaccine names used in the United States have well-established abbreviations that are also widely known and used elsewhere. An extensive list of them provided in a sortable table and freely accessible is available at a USCenters for Disease Control and Preventionweb page.[101]The page explains that "The abbreviations [in] this table (Column 3) were standardized jointly by staff of the Centers for Disease Control and Prevention,ACIPWork Groups, the editor of theMorbidity and Mortality Weekly Report(MMWR), the editor ofEpidemiology and Prevention of Vaccine-Preventable Diseases(the Pink Book), ACIP members, and liaison organizations to the ACIP. "[101]
Some examples are "DTaP"for diphtheria and tetanus toxoids and acellular pertussis vaccine," DT "for diphtheria and tetanus toxoids, and" Td "for tetanus and diphtheria toxoids. At its page on tetanus vaccination,[102]the CDC further explains that "Upper-case letters in these abbreviations denote full-strength doses of diphtheria (D) and tetanus (T) toxoids and pertussis (P) vaccine. Lower-case" d "and" p "denote reduced doses of diphtheria and pertussis used in the adolescent/adult-formulations. The 'a' in DTaP and Tdap stands for 'acellular', meaning that the pertussis component contains only a part of the pertussis organism."[102]
Another list of established vaccine abbreviations is at the CDC's page called "Vaccine Acronyms and Abbreviations", with abbreviations used on U.S. immunization records.[103]TheUnited States Adopted Namesystem has some conventions for theword orderof vaccine names, placinghead nounsfirst andadjectives postpositively.This is why the USAN for "OPV"is" poliovirus vaccine live oral "rather than" oral poliovirus vaccine ".
Licensing
A vaccinelicensureoccurs after the successful conclusion of the development cycle and further the clinical trials and other programs involved throughPhasesI–III demonstrating safety, immunoactivity, immunogenetic safety at a given specific dose, proven effectiveness in preventing infection for target populations, and enduring preventive effect (time endurance or need for revaccination must be estimated).[104]Because preventive vaccines are predominantly evaluated in healthy population cohorts and distributed among the general population, a high standard of safety is required.[105]As part of a multinational licensing of a vaccine, the World Health OrganizationExpert Committee on Biological Standardizationdeveloped guidelines of international standards for manufacturing andquality controlof vaccines, a process intended as a platform for national regulatory agencies to apply for their own licensing process.[104]Vaccine manufacturers do not receive licensing until a complete clinical cycle of development and trials proves the vaccine is safe and has long-term effectiveness, following scientific review by a multinational or national regulatory organization, such as theEuropean Medicines Agency(EMA) or the USFood and Drug Administration(FDA).[106][107]
Upondeveloping countriesadopting WHO guidelines for vaccine development and licensure, each country has its own responsibility to issue a national licensure, and to manage, deploy, and monitor the vaccine throughout its use in each nation.[104]Building trust and acceptance of a licensed vaccine among the public is a task of communication by governments and healthcare personnel to ensure a vaccination campaign proceeds smoothly, saves lives, and enables economic recovery.[108][109]When a vaccine is licensed, it will initially be in limited supply due to variable manufacturing, distribution, and logistical factors, requiring an allocation plan for the limited supply and which population segments should be prioritized to first receive the vaccine.[108]
World Health Organization
Vaccines developed for multinational distribution via theUnited Nations Children's Fund (UNICEF)require pre-qualification by the WHO to ensureinternational standardsof quality, safety, immunogenicity, and efficacy for adoption by numerous countries.[104]
The process requires manufacturing consistency at WHO-contracted laboratories followingGood Manufacturing Practice(GMP).[104]When UN agencies are involved in vaccine licensure, individual nations collaborate by 1) issuing marketing authorization and a national license for the vaccine, its manufacturers, and distribution partners; and 2) conductingpostmarketing surveillance,including records for adverse events after the vaccination program. The WHO works with national agencies to monitor inspections of manufacturing facilities and distributors for compliance with GMP and regulatory oversight.[104]
Some countries choose to buy vaccines licensed by reputable national organizations, such as EMA, FDA, or national agencies in other affluent countries, but such purchases typically are more expensive and may not have distribution resources suitable to local conditions in developing countries.[104]
European Union
In the European Union (EU), vaccines for pandemic pathogens, such asseasonal influenza,are licensed EU-wide where all themember statescomply ( "centralized" ), are licensed for only some member states ( "decentralized" ), or are licensed on an individual national level.[106]Generally, all EU states follow regulatory guidance and clinical programs defined by the EuropeanCommittee for Medicinal Products for Human Use(CHMP), a scientific panel of theEuropean Medicines Agency(EMA) responsible for vaccine licensure.[106]The CHMP is supported by several expert groups who assess and monitor the progress of a vaccine before and after licensure and distribution.[106]
United States
Under the FDA, the process of establishing evidence for vaccine clinical safety and efficacy is the same as forthe approval process for prescription drugs.[110]If successful through the stages of clinical development, the vaccine licensing process is followed by aBiologics License Applicationwhich must provide a scientific review team (from diverse disciplines, such as physicians, statisticians, microbiologists, chemists) and comprehensive documentation for the vaccine candidate having efficacy and safety throughout its development. Also during this stage, the proposed manufacturing facility is examined by expert reviewers for GMP compliance, and the label must have a compliant description to enable health care providers' definition of vaccine-specific use, including its possible risks, to communicate and deliver the vaccine to the public.[110]After licensure, monitoring of the vaccine and its production, including periodic inspections for GMP compliance, continue as long as the manufacturer retains its license, which may include additional submissions to the FDA of tests for potency, safety, and purity for each vaccine manufacturing step.[110]
India
In India, theDrugs Controller General,the head of department of theCentral Drugs Standard Control Organization,India's national regulatory body for cosmetics, pharmaceuticals and medical devices, is responsible for the approval of licences for specified categories of drugs such as vaccines and other medicinal items, such as blood or blood products, IV fluids, and sera.[111]
Postmarketing surveillance
Until a vaccine is in use amongst the general population, all potentialadverse eventsfrom the vaccine may not be known, requiring manufacturers to conductPhaseIVstudies forpostmarketing surveillanceof the vaccine while it is used widely in the public.[104][110]The WHO works with UN member states to implement post-licensing surveillance.[104]The FDA relies on aVaccine Adverse Event Reporting Systemto monitor safety concerns about a vaccine throughout its use in the American public.[110]
Scheduling
In order to provide the best protection, children are recommended to receive vaccinations as soon as their immune systems are sufficiently developed to respond to particular vaccines, with additional "booster" shots often required to achieve "full immunity". This has led to the development of complex vaccination schedules. Global recommendations of vaccination schedule are issued byStrategic Advisory Group of Expertsand will be further translated byadvisory committeeat the country level with considering of local factors such as disease epidemiology, acceptability of vaccination, equity in local populations, and programmatic and financial constraint.[112]In the United States, theAdvisory Committee on Immunization Practices,which recommends schedule additions for theCenters for Disease Control and Prevention,recommends routine vaccination of children against[113]hepatitis A,hepatitis B,polio, mumps, measles, rubella,diphtheria,pertussis,tetanus,HiB,chickenpox,rotavirus,influenza,meningococcal diseaseandpneumonia.[114]
The large number of vaccines and boosters recommended (up to 24 injections by age two) has led to problems with achieving full compliance. To combat declining compliance rates, various notification systems have been instituted and many combination injections are now marketed (e.g.,Pentavalent vaccineandMMRV vaccine), which protect against multiple diseases.
Besides recommendations for infant vaccinations and boosters, many specific vaccines are recommended for other ages or for repeated injections throughout life – most commonly for measles, tetanus, influenza, and pneumonia. Pregnant women are often screened for continued resistance to rubella. Thehuman papillomavirusvaccine is recommended in the U.S. (as of 2011)[115]and UK (as of 2009).[116]Vaccine recommendations for the elderly concentrate on pneumonia and influenza, which are more deadly to that group. In 2006, a vaccine was introduced againstshingles,a disease caused by the chickenpox virus, which usually affects the elderly.[117]
Scheduling and dosing of a vaccination may be tailored to the level of immunocompetence of an individual[118]and to optimize population-wide deployment of a vaccine when it supply is limited,[119]e.g. in the setting of a pandemic.
Economics of development
One challenge in vaccine development is economic: Many of the diseases most demanding a vaccine, includingHIV,malariaand tuberculosis, exist principally in poor countries. Pharmaceutical firms andbiotechnologycompanies have little incentive to develop vaccines for these diseases because there is little revenue potential. Even in more affluent countries, financial returns are usually minimal and the financial and other risks are great.[120]
Most vaccine development to date has relied on "push" funding by government, universities and non-profit organizations.[121]Many vaccines have been highly cost effective and beneficial forpublic health.[122]The number of vaccines actually administered has risen dramatically in recent decades.[123]This increase, particularly in the number of different vaccines administered to children before entry into schools may be due to government mandates and support, rather than economic incentive.[124]
Patents
According to the World Health Organization, the biggest barrier to vaccine production in less developed countries has not beenpatents,but the substantial financial,infrastructure,and workforce requirements needed for market entry. Vaccines are complex mixtures of biological compounds, and unlike the case forprescription drugs,there are no truegeneric vaccines.The vaccine produced by a new facility must undergo complete clinical testing for safety and efficacy by the manufacturer. For most vaccines, specific processes in technology are patented. These can be circumvented by alternative manufacturing methods, but this required R&D infrastructure and a suitably skilled workforce. In the case of a few relatively new vaccines, such as thehuman papillomavirusvaccine, the patents may impose an additional barrier.[125]
When increased production of vaccines was urgently needed during theCOVID-19 pandemicin 2021, theWorld Trade Organizationand governments around the world evaluated whether to waiveintellectual propertyrights and patents onCOVID-19 vaccines,which would "eliminate all potential barriers to the timely access of affordable COVID-19 medical products, including vaccines and medicines, and scale up the manufacturing and supply of essential medical products".[126]
Production
Vaccine production is fundamentally different from other kinds of manufacturing – including regularpharmaceutical manufacturing– in that vaccines are intended to be administered to millions of people of whom the vast majority are perfectly healthy.[127]This fact drives an extraordinarily rigorous production process with strict compliance requirements that go far beyond what is required of other products.[127]
Depending upon the antigen, it can cost anywhere from US$50 to $500 million to build a vaccine production facility, which requires highly specialized equipment,clean rooms,and containment rooms.[128]There is a global scarcity of personnel with the right combination of skills, expertise, knowledge, competence and personality to staff vaccine production lines.[128]With the notable exceptions of Brazil, China, and India, many developing countries' educational systems are unable to provide enough qualified candidates, and vaccine makers based in such countries must hire expatriate personnel to keep production going.[128]
Vaccine production has several stages. First, the antigen itself is generated. Viruses are grown either on primary cells such aschicken eggs(e.g., for influenza) or on continuous cell lines such as cultured human cells (e.g., forhepatitis A).[129]Bacteria are grown inbioreactors(e.g.,Haemophilus influenzaetype b). Likewise, a recombinant protein derived from the viruses or bacteria can be generated in yeast, bacteria, or cell cultures.[130][131]
After the antigen is generated, it is isolated from the cells used to generate it. A virus may need to be inactivated, possibly with no further purification required. Recombinant proteins need many operations involving ultrafiltration and column chromatography. Finally, the vaccine is formulated by adding adjuvant, stabilizers, and preservatives as needed. The adjuvant enhances the immune response to the antigen, stabilizers increase the storage life, and preservatives allow the use of multidose vials.[130][131]Combination vaccines are harder to develop and produce, because of potential incompatibilities and interactions among the antigens and other ingredients involved.[132]
The final stage in vaccine manufacture before distribution isfill and finish,which is the process of filling vials with vaccines and packaging them for distribution. Although this is a conceptually simple part of the vaccine manufacture process, it is often a bottleneck in the process of distributing and administering vaccines.[133][134][135]
Vaccine production techniques are evolving. Culturedmammalian cellsare expected to become increasingly important, compared to conventional options such as chicken eggs, due to greater productivity and low incidence of problems with contamination. Recombination technology that produces genetically detoxified vaccines is expected to grow in popularity for the production of bacterial vaccines that use toxoids. Combination vaccines are expected to reduce the quantities of antigens they contain, and thereby decrease undesirable interactions, by usingpathogen-associated molecular patterns.[132]
Vaccine manufacturers
The companies with the highest market share in vaccine production areMerck,Sanofi,GlaxoSmithKline,PfizerandNovartis,with 70% of vaccine sales concentrated in the EU or US (2013).[136]: 42 Vaccine manufacturing plants require large capital investments ($50 million up to $300 million) and may take between 4 and 6 years to construct, with the full process of vaccine development taking between 10 and 15 years.[136]: 43 Manufacturing in developing countries is playing an increasing role in supplying these countries, specifically with regards to older vaccines and in Brazil, India and China.[136]: 47 The manufacturers in India are the most advanced in the developing world and include theSerum Institute of India,one of the largest producers of vaccines by number of doses and an innovator in processes, recently improving efficiency of producing the measles vaccine by 10 to 20-fold, due to switching to aMRC-5cell culture instead of chicken eggs.[136]: 48 China's manufacturing capabilities are focused on supplying their own domestic need, withSinopharm (CNPGC)alone providing over 85% of the doses for 14 different vaccines in China.[136]: 48 Brazil is approaching the point of supplying its own domestic needs using technology transferred from the developed world.[136]: 49
Delivery systems
One of the most common methods of delivering vaccines into the human body isinjection.
The development of new delivery systems raises the hope of vaccines that are safer and more efficient to deliver and administer. Lines of research includeliposomesandISCOM(immune stimulating complex).[137]
Notable developments in vaccine delivery technologies have included oral vaccines. Early attempts to apply oral vaccines showed varying degrees of promise, beginning early in the 20th century, at a time when the very possibility of an effective oral antibacterial vaccine was controversial.[138]By the 1930s there was increasing interest in the prophylactic value of an oraltyphoid fevervaccine for example.[139]
Anoral polio vaccineturned out to be effective when vaccinations were administered by volunteer staff without formal training; the results also demonstrated increased ease and efficiency of administering the vaccines. Effective oral vaccines have many advantages; for example, there is no risk of blood contamination. Vaccines intended for oral administration need not be liquid, and as solids, they commonly are more stable and less prone to damage or spoilage by freezing in transport and storage.[140]Such stability reduces the need for a "cold chain":the resources required to keep vaccines within a restricted temperature range from the manufacturing stage to the point of administration, which, in turn, may decrease costs of vaccines.
A microneedle approach, which is still in stages of development, uses "pointed projections fabricated into arrays that can create vaccine delivery pathways through the skin".[141]
An experimental needle-free[142]vaccine delivery system is undergoing animal testing.[143][144]A stamp-size patch similar to anadhesive bandagecontains about 20,000 microscopic projections per square cm.[145]Thisdermaladministration potentially increases the effectiveness of vaccination, while requiring less vaccine than injection.[146]
In veterinary medicine
Vaccinations of animals are used both to prevent their contracting diseases and to prevent transmission of disease to humans.[147]Both animals kept as pets and animals raised as livestock are routinely vaccinated. In some instances, wild populations may be vaccinated. This is sometimes accomplished with vaccine-laced food spread in a disease-prone area and has been used to attempt to controlrabiesinraccoons.
Where rabies occurs, rabies vaccination of dogs may be required by law. Other canine vaccines includecanine distemper,canine parvovirus,infectious canine hepatitis,adenovirus-2,leptospirosis,Bordetella,canineparainfluenza virus,andLyme disease,among others.
Cases of veterinary vaccines used in humans have been documented, whether intentional or accidental, with some cases of resultant illness, most notably withbrucellosis.[148]However, the reporting of such cases is rare and very little has been studied about the safety and results of such practices. With the advent of aerosol vaccination in veterinary clinics, human exposure to pathogens not naturally carried in humans, such asBordetella bronchiseptica,has likely increased in recent years.[148]In some cases, most notablyrabies,the parallel veterinary vaccine against a pathogen may be as much asorders of magnitudemore economical than the human one.
DIVA vaccines
DIVA (Differentiation of Infected from Vaccinated Animals), also known as SIVA (Segregation of Infected from Vaccinated Animals) vaccines, make it possible to differentiate between infected and vaccinated animals. DIVA vaccines carry at least oneepitopeless than the equivalent wild microorganism. An accompanying diagnostic test that detects the antibody against that epitope assists in identifying whether the animal has been vaccinated or not.[citation needed]
The first DIVA vaccines (formerly termedmarker vaccinesand since 1999 coined as DIVA vaccines) and companion diagnostic tests were developed by J. T. van Oirschot and colleagues at the Central Veterinary Institute in Lelystad, The Netherlands.[149][150]They found that some existing vaccines againstpseudorabies(also termed Aujeszky's disease) had deletions in their viral genome (among which was the gE gene). Monoclonal antibodies were produced against that deletion and selected to develop anELISAthat demonstrated antibodies against gE. In addition, novel genetically engineered gE-negative vaccines were constructed.[151]Along the same lines, DIVA vaccines and companion diagnostic tests against bovine herpesvirus1 infections have been developed.[150][152]
The DIVA strategy has been applied in various countries to successfully eradicate pseudorabies virus from those countries. Swine populations were intensively vaccinated and monitored by the companion diagnostic test and, subsequently, the infected pigs were removed from the population. Bovine herpesvirus1 DIVA vaccines are also widely used in practice.[citation needed]Considerable efforts are ongoing to apply the DIVA principle to a wide range of infectious diseases, such as classical swine fever,[153]avian influenza,[154]Actinobacillus pleuropneumonia[155]andSalmonellainfections in pigs.[156]
History
Prior to the introduction of vaccination with material from cases of cowpox (heterotypic immunisation), smallpox could be prevented by deliberatevariolationwith smallpox virus. The earliest hints of the practice of variolation for smallpox in China come during the tenth century.[157][further explanation needed]The Chinese also practiced the oldest documented use of variolation, dating back to the fifteenth century. They implemented a method of "nasalinsufflation"administered by blowing powdered smallpox material, usually scabs, up the nostrils. Various insufflation techniques have been recorded throughout the sixteenth and seventeenth centuries within China.[158]: 60 Two reports on the Chinese practice ofinoculationwere received by theRoyal Societyin London in 1700; one byMartin Listerwho received a report by an employee of theEast India Companystationed in China and another byClopton Havers.[159]In France,Voltairereports that the Chinese have practiced variolation "these hundred years".[160]
Mary Wortley Montagu,who had witnessed variolation in Turkey, had her four-year-old daughter variolated in the presence ofphysiciansof the Royal Court in 1721 upon her return to England.[158]Later on that year,Charles Maitlandconducted an experimental variolation of six prisoners inNewgate Prisonin London.[161]The experiment was a success, and soon variolation was drawing attention from the royal family, who helped promote the procedure. However, in 1783, several days afterPrince Octavius of Great Britainwas inoculated, he died.[162]In 1796, the physicianEdward Jennertook pus from the hand of a milkmaid withcowpox,scratched it into the arm of an 8-year-old boy,James Phipps,and six weeks later variolated the boy with smallpox, afterwards observing that he did not catch smallpox.[163][164]Jenner extended his studies and, in 1798, reported that his vaccine was safe in children and adults, and could be transferred from arm-to-arm, which reduced reliance on uncertain supplies from infected cows.[162]In 1804, the SpanishBalmis smallpox vaccination expeditionto Spain's colonies Mexico and Philippines used the arm-to-arm transport method to get around the fact the vaccine survived for only 12 daysin vitro.They used cowpox.[165]Since vaccination with cowpox was much safer than smallpox inoculation,[166]the latter, though still widely practiced in England, was banned in 1840.[167]
Following on from Jenner's work, the second generation of vaccines was introduced in the 1880s byLouis Pasteurwho developed vaccines forchicken choleraandanthrax,[17]and from the late nineteenth century vaccines were considered a matter of national prestige. Nationalvaccination policieswere adopted and compulsory vaccination laws were passed.[163]In 1931Alice Miles WoodruffandErnest Goodpasturedocumented that thefowlpoxvirus could be grown inembryonatedchickenegg.Soon scientists began cultivating other viruses in eggs. Eggs were used for virus propagation in the development of ayellow fever vaccinein 1935 and aninfluenza vaccinein 1945. In 1959growth mediaandcell culturereplaced eggs as the standard method of virus propagation for vaccines.[168]
Vaccinology flourished in the twentieth century, which saw the introduction of several successful vaccines, including those againstdiphtheria,measles,mumps,andrubella.Major achievements included the development of thepolio vaccinein the 1950s and theeradication of smallpoxduring the 1960s and 1970s.Maurice Hillemanwas the most prolific of the developers of the vaccines in the twentieth century. As vaccines became more common, many people began taking them for granted. However, vaccines remain elusive for many important diseases, includingherpes simplex,malaria,gonorrhea,andHIV.[163][169]
Generations of vaccines
First generation vaccines are whole-organism vaccines – either live andweakened,or killed forms.[170]Live, attenuated vaccines, such as smallpox and polio vaccines, are able to inducekiller T-cell(TCor CTL) responses,helper T-cell(TH) responses and antibodyimmunity.However, attenuated forms of apathogencan convert to a dangerous form and may cause disease inimmunocompromisedvaccine recipients (such as those withAIDS). While killed vaccines do not have this risk, they cannot generate specific killer T-cell responses and may not work at all for some diseases.[170]
Second generation vaccines were developed to reduce the risks from live vaccines. These are subunit vaccines, consisting of specificproteinantigens (such astetanusordiphtheriatoxoid) orrecombinantprotein components (such as the hepatitis B surfaceantigen). They can generate THandantibodyresponses, but not killer T cell responses.[citation needed]
RNA vaccinesandDNA vaccinesare examples of third generation vaccines.[170][171][172]In 2016 a DNA vaccine for theZika virusbegan testing at theNational Institutes of Health.Separately, Inovio Pharmaceuticals and GeneOne Life Science began tests of a different DNA vaccine against Zika in Miami. Manufacturing the vaccines in volume was unsolved as of 2016.[173]Clinical trials for DNA vaccines to prevent HIV are underway.[174]mRNA vaccinessuch asBNT162b2were developed in the year 2020 with the help ofOperation Warp Speedand massively deployed to combat theCOVID-19 pandemic.In 2021,Katalin KarikóandDrew Weissmanreceived Columbia University's Horwitz Prize for their pioneering research in mRNA vaccine technology.[175]
Trends
This section needs to beupdated.(June 2018) |
Since at least 2013, scientists have been trying to develop synthetic third-generation vaccines by reconstructing the outside structure of avirus;it was hoped that this will help preventvaccine resistance.[176]
Principles that govern the immune response can now be used in tailor-made vaccines against many noninfectious human diseases, such as cancers and autoimmune disorders.[177]For example, the experimental vaccineCYT006-AngQbhas been investigated as a possible treatment forhigh blood pressure.[178]Factors that affect the trends of vaccine development include progress in translatory medicine,demographics,regulatory science,political, cultural, and social responses.[179]
Plants as bioreactors for vaccine production
The idea of vaccine production viatransgenic plantswas identified as early as 2003. Plants such astobacco,potato,tomato,andbananacan have genes inserted that cause them to produce vaccines usable for humans.[180]In 2005, bananas were developed that produce a human vaccine againsthepatitis B.[181]
Vaccine hesitancy
Vaccine hesitancyis a delay in acceptance, or refusal of vaccines despite the availability of vaccine services. The term covers outright refusals to vaccinate, delaying vaccines, accepting vaccines but remaining uncertain about their use, or using certain vaccines but not others.[183][184][185][186]There is an overwhelmingscientific consensusthat vaccines are generally safe and effective.[187][188][189][190]Vaccine hesitancy often results in diseaseoutbreaksand deaths fromvaccine-preventable diseases.[191][192][193][194][195][196]TheWorld Health Organizationtherefore characterized vaccine hesitancy as one of the top ten global health threats in 2019.[197][198]
See also
- Biologics Control Act
- Coalition for Epidemic Preparedness Innovations
- Flying syringe
- Immunization registry
- Immunotherapy
- List of vaccine ingredients
- List of vaccine topics
- Non-specific effect of vaccines
- OPV AIDS hypothesis
- Preventive healthcare
- Reverse vaccinology
- TA-CD
- Timeline of vaccines
- Virosome
- Vaccinator
- Vaccine adverse event(safety issues)
- Vaccine cooler
- Vaccine failure
- Vaccine hesitancy
- Vaccinov
- Viral vector
- Virus-like particle
- Nasal vaccine
References
- ^"Expanded Practice Standards"(PDF).Iowa Administrative Code.2019.Archived(PDF)from the original on 2023-01-19.Retrieved2023-01-16.
- ^"Immunization: The Basics".Centers for Disease Control and Prevention.22 November 2022.Archivedfrom the original on 12 July 2023.RetrievedJuly 8,2023.
- ^Amanna, Ian J.; Slifka, Mark K. (2018). "Successful Vaccines". In Lars Hangartner; Dennis R. Burton (eds.).Vaccination Strategies Against Highly Variable Pathogens.Current Topics in Microbiology and Immunology, vol. 428. Vol. 428. Springer. pp. 1–30.doi:10.1007/82_2018_102.ISBN978-3-030-58003-2.PMC6777997.PMID30046984.
The effect of vaccines on public health is truly remarkable. One study examining the impact of childhood vaccination on the 2001 US birth cohort found that vaccines prevented 33,000 deaths and 14 million cases of disease (Zhou et al. 2005). Among 73 nations supported by the GAVI alliance, mathematical models project that vaccines will prevent 23.3 million deaths from 2011–2020 compared to what would have occurred if there were no vaccines available (Lee et al. 2013). Vaccines have been developed against a wide assortment of human pathogens.
- ^Zimmer, Carl(20 November 2020)."2 Companies Say Their Vaccines Are 95% Effective. What Does That Mean? You might assume that 95 out of every 100 people vaccinated will be protected from Covid-19. But that's not how the math works".The New York Times.Archivedfrom the original on 22 November 2020.Retrieved21 November2020.
- ^Melief CJ, van Hall T, Arens R, Ossendorp F, van der Burg SH (September 2015)."Therapeutic cancer vaccines".The Journal of Clinical Investigation.125(9): 3401–3412.doi:10.1172/JCI80009.PMC4588240.PMID26214521.
- ^Bol KF, Aarntzen EH, Pots JM, Olde Nordkamp MA, van de Rakt MW, Scharenborg NM, de Boer AJ, van Oorschot TG, Croockewit SA, Blokx WA, Oyen WJ, Boerman OC, Mus RD, van Rossum MM, van der Graaf CA, Punt CJ, Adema GJ, Figdor CG, de Vries IJ, Schreibelt G (March 2016)."Prophylactic vaccines are potent activators of monocyte-derived dendritic cells and drive effective anti-tumor responses in melanoma patients at the cost of toxicity".Cancer Immunology, Immunotherapy.65(3): 327–339.doi:10.1007/s00262-016-1796-7.PMC4779136.PMID26861670.
- ^Brotherton J (2015). "HPV prophylactic vaccines: lessons learned from 10 years experience".Future Virology.10(8): 999–1009.doi:10.2217/fvl.15.60.
- ^Frazer IH (May 2014)."Development and implementation of papillomavirus prophylactic vaccines".Journal of Immunology.192(9): 4007–4011.doi:10.4049/jimmunol.1490012.PMID24748633.
- ^Ledford, Heidi (2020-08-17)."What the immune response to the coronavirus says about the prospects for a vaccine".Nature.585(7823): 20–21.Bibcode:2020Natur.585...20L.doi:10.1038/d41586-020-02400-7.PMID32811981.S2CID221180503.
- ^*United States Centers for Disease Control and Prevention (2011).A CDC framework for preventing infectious diseases.Archived2017-08-29 at theWayback MachineAccessed 11 September 2012. "Vaccines are our most effective and cost-saving tools for disease prevention, preventing untold suffering and saving tens of thousands of lives and billions of dollars in healthcare costs each year."
- American Medical Association (2000).Vaccines and infectious diseases: putting risk into perspective.Archived2015-02-05 at theWayback MachineAccessed 11 September 2012. "Vaccines are the most effective public health tool ever created."
- Public Health Agency of Canada.Vaccine-preventable diseases.Archived2015-03-13 at theWayback MachineAccessed 11 September 2012. "Vaccines still provide the most effective, longest-lasting method of preventing infectious diseases in all age groups."
- United States National Institute of Allergy and Infectious Diseases (NIAID).NIAID Biodefense Research Agenda for Category B and C Priority Pathogens.Archived2016-03-04 at theWayback MachineAccessed 11 September 2012. "Vaccines are the most effective method of protecting the public against infectious diseases."
- ^World Health Organization,Global Vaccine Action Plan 2011-2020.Archived2014-04-14 at theWayback MachineGeneva, 2012.
- ^Williams 2010,p. 60.
- ^Lombard M, Pastoret PP, Moulin AM (April 2007)."A brief history of vaccines and vaccination".Revue Scientifique et Technique.26(1): 29–48.doi:10.20506/rst.26.1.1724.PMID17633292.S2CID6688481.
- ^Behbehani AM (December 1983)."The smallpox story: life and death of an old disease".Microbiological Reviews.47(4): 455–509.doi:10.1128/MMBR.47.4.455-509.1983.PMC281588.PMID6319980.
- ^Ferguson, Donna (28 March 2021)."How Mary Wortley Montagu's bold experiment led to smallpox vaccine – 75 years before Jenner".the Guardian.Archivedfrom the original on 11 July 2022.Retrieved11 July2022.
- ^Baxby D(January 1999). "Edward Jenner's Inquiry; a bicentenary analysis".Vaccine.17(4): 301–307.doi:10.1016/s0264-410x(98)00207-2.PMID9987167.
- ^abPasteur L (1881). "Address on the Germ Theory".Lancet.118(3024): 271–272.doi:10.1016/s0140-6736(02)35739-8.
- ^"Measles Vaccination CDC".2018-02-05.Archivedfrom the original on 2019-11-19.Retrieved2018-11-13.
- ^Orenstein WA, Bernier RH, Dondero TJ, Hinman AR, Marks JS, Bart KJ, Sirotkin B (1985)."Field evaluation of vaccine efficacy".Bulletin of the World Health Organization.63(6): 1055–1068.PMC2536484.PMID3879673.
- ^"The science is clear: Vaccines are safe, effective, and do not cause autism".The Hub.2017-01-11.Archivedfrom the original on 2017-09-28.Retrieved2019-04-16.
- ^Ellenberg SS, Chen RT (1997)."The complicated task of monitoring vaccine safety".Public Health Reports.112(1): 10–20, discussion 21.PMC1381831.PMID9018282.
- ^"Vaccine Safety: The Facts".HealthyChildren.org.Archivedfrom the original on 2019-04-16.Retrieved2019-04-16.
- ^Mak, Tak W.; Saunders, Mary E.; Jett, Bradley D. (2014)."Chapter 1 - Introduction to the Immune Response".Primer to The immune response(2nd ed.). Burlington, MA: Academic Cell. pp. 3–20.ISBN978-0-12-385245-8.Archivedfrom the original on 18 April 2022.Retrieved18 April2022.
- ^Clem, Angela S (2011)."Fundamentals of Vaccine Immunology".Journal of Global Infectious Diseases.3(1): 73–78.doi:10.4103/0974-777X.77299.ISSN0974-777X.PMC3068582.PMID21572612.
- ^Grammatikos AP, Mantadakis E, Falagas ME (June 2009). "Meta-analyses on pediatric infections and vaccines".Infectious Disease Clinics of North America.23(2): 431–457.doi:10.1016/j.idc.2009.01.008.PMID19393917.
- ^abcdWiedermann U, Garner-Spitzer E, Wagner A (2016)."Primary vaccine failure to routine vaccines: Why and what to do?".Human Vaccines & Immunotherapeutics.12(1): 239–243.doi:10.1080/21645515.2015.1093263.ISSN2164-554X.PMC4962729.PMID26836329.
- ^Justiz Vaillant, AA; Ramphul, K (January 2022).Antibody Deficiency Disorder.Treasure Island, FL: StatPearls Publishing.PMID29939682.Retrieved18 April2022.
- ^Reda, Shereen M.; Cant, Andrew J. (May 2015). "The importance of vaccination and immunoglobulin treatment for patients with primary immunodeficiency diseases (PIDs) – World PI Week April 22–29, 2015: FORUM".European Journal of Immunology.45(5): 1285–1286.doi:10.1002/eji.201570054.PMID25952627.S2CID1922332.
- ^Jo, Eun-Kyeong (December 2019)."Interplay between host and pathogen: immune defense and beyond".Experimental & Molecular Medicine.51(12): 1–3.doi:10.1038/s12276-019-0281-8.ISSN2092-6413.PMC6906370.PMID31827066.
- ^abJaneway, Charles A Jr.; Travers, Paul; Walport, Mark; Shlomchik, Mark J. (2001)."The Humoral Immune Response".Immunobiology: The Immune System in Health and Disease(5th ed.).Archivedfrom the original on 2 January 2021.Retrieved18 April2022.
- ^Grubbs, Hailey; Kahwaji, Chadi I. (January 2022).Physiology, Active Immunity.Treasure Island, FL: StatPearls Publishing.PMID29939682.Archivedfrom the original on 12 November 2021.Retrieved18 April2022.
- ^Gomes, M. Gabriela M.; White, Lisa J.; Medley, Graham F. (21 June 2004). "Infection, reinfection, and vaccination under suboptimal immune protection: epidemiological perspectives".Journal of Theoretical Biology.228(4): 539–549.Bibcode:2004JThBi.228..539G.doi:10.1016/j.jtbi.2004.02.015.hdl:10400.7/53.ISSN0022-5193.PMID15178201.
- ^Bonanni, Paolo; Picazo, Juan José; Rémy, Vanessa (12 August 2015)."The intangible benefits of vaccination – what is the true economic value of vaccination?".Journal of Market Access & Health Policy.3:10.3402/jmahp.v3.26964.doi:10.3402/jmahp.v3.26964.ISSN2001-6689.PMC4802696.PMID27123182.
- ^Stanciu, Stefan G. (24 August 2016).Micro and Nanotechnologies for Biotechnology.BoD – Books on Demand.ISBN978-953-51-2530-3.Archivedfrom the original on 14 January 2023.Retrieved19 April2022.
- ^Frasca, Daniela; Diaz, Alain; Romero, Maria; Garcia, Denisse; Blomberg, Bonnie B. (6 October 2020)."B Cell Immunosenescence".Annual Review of Cell and Developmental Biology.36(1): 551–574.doi:10.1146/annurev-cellbio-011620-034148.ISSN1081-0706.PMC8060858.PMID33021823.
- ^Neighmond P (2010-02-07)."Adapting Vaccines For Our Aging Immune Systems".Morning Edition.NPR.Archivedfrom the original on 2013-12-16.Retrieved2014-01-09.
- ^Schlegel M, Osterwalder JJ, Galeazzi RL, Vernazza PL (August 1999)."Comparative efficacy of three mumps vaccines during disease outbreak in Eastern Switzerland: cohort study".BMJ.319(7206): 352.doi:10.1136/bmj.319.7206.352.PMC32261.PMID10435956.
- ^Préziosi MP, Halloran ME (September 2003)."Effects of pertussis vaccination on disease: vaccine efficacy in reducing clinical severity".Clinical Infectious Diseases.37(6): 772–779.doi:10.1086/377270.PMID12955637.
- ^Miller, E.; Beverley, P. C. L.; Salisbury, D. M. (2002-07-01)."Vaccine programmes and policies".British Medical Bulletin.62(1): 201–211.doi:10.1093/bmb/62.1.201.ISSN0007-1420.PMID12176861.
- ^Orenstein WA, Papania MJ, Wharton ME (May 2004)."Measles elimination in the United States".The Journal of Infectious Diseases.189(Suppl 1): S1–3.doi:10.1086/377693.PMID15106120.
- ^abc"Measles – United States, January 1 – April 25, 2008".MMWR. Morbidity and Mortality Weekly Report.57(18): 494–498. May 2008.PMID18463608.Archivedfrom the original on October 11, 2017.
- ^"WHO | Smallpox".WHO.World Health Organization.Archivedfrom the original on 2007-09-22.Retrieved2019-04-16.
- ^"WHO South-East Asia Region certified polio-free".WHO. 27 March 2014. Archived fromthe originalon 27 March 2014.RetrievedNovember 3,2014.
- ^"Statement following the Twenty-Eighth IHR Emergency Committee for Polio".World Health Organization.21 May 2021.Archivedfrom the original on 19 April 2022.Retrieved19 April2022.
- ^Grassly, Nicholas C. (5 August 2013)."The final stages of the global eradication of poliomyelitis".Philosophical Transactions of the Royal Society B: Biological Sciences.368(1623): 20120140.doi:10.1098/rstb.2012.0140.ISSN0962-8436.PMC3720038.PMID23798688.
- ^Ittefaq, Muhammad; Abwao, Mauryne; Rafique, Shanawer (3 August 2021)."Polio vaccine misinformation on social media: turning point in the fight against polio eradication in Pakistan".Human Vaccines & Immunotherapeutics.17(8): 2575–2577.doi:10.1080/21645515.2021.1894897.ISSN2164-554X.PMC8475597.PMID33705246.
- ^"Disinformation disturbs anti-polio drives".The Express Tribune.24 January 2022.Archivedfrom the original on 10 May 2022.Retrieved19 April2022.
- ^"19 July 2017Vaccines promoted as key to stamping out drug-resistant microbes"Immunization can stop resistant infections before they get started, say scientists from industry and academia."".Archived fromthe originalon July 22, 2017.
- ^Sullivan P (2005-04-13)."Maurice R. Hilleman dies; created vaccines".Wash. Post.Archivedfrom the original on 2012-10-20.Retrieved2014-01-09.
- ^Dudley, Matthew Z; Halsey, Neal A; Omer, Saad B; Orenstein, Walter A; O'Leary, Sean T; Limaye, Rupali J; Salmon, Daniel A (May 2020). "The state of vaccine safety science: systematic reviews of the evidence".The Lancet Infectious Diseases.20(5): e80–e89.doi:10.1016/s1473-3099(20)30130-4.ISSN1473-3099.PMID32278359.S2CID215751248.
- ^abcdMaglione MA, Das L, Raaen L, Smith A, Chari R, Newberry S, Shanman R, Perry T, Goetz MB, Gidengil C (August 2014)."Safety of vaccines used for routine immunization of U.S. children: a systematic review".Pediatrics.134(2): 325–337.doi:10.1542/peds.2014-1079.PMID25086160.Archivedfrom the original on 2020-01-30.Retrieved2019-07-01.
- ^abc"Possible Side-effects from Vaccines".Centers for Disease Control and Prevention.2018-07-12.Archivedfrom the original on 17 March 2017.Retrieved24 February2014.
- ^"Seasonal Flu Shot – Seasonal Influenza".CDC. 2018-10-02. Archived fromthe originalon 2015-10-01.Retrieved2017-09-17.
- ^Looker C, Heath K (2011)."No-fault compensation following adverse events attributed to vaccination: a review of international programmes".Bulletin of the World Health Organization.89(5). Word Health Organisation: 371–378.doi:10.2471/BLT.10.081901.PMC3089384.PMID21556305.Archived fromthe originalon August 11, 2013.
- ^"Vaccine Types".National Institute of Allergy and Infectious Diseases.2012-04-03.Archivedfrom the original on 2015-09-05.Retrieved2015-01-27.
- ^Sinha JK, Bhattacharya S.A Text Book of Immunology(Google Books Preview).Academic Publishers. p. 318.ISBN978-81-89781-09-5.Retrieved2014-01-09.
- ^"Types of Vaccines".Archivedfrom the original on 2017-07-29.RetrievedOctober 19,2017.
- ^Batah, Aly; Ahmad, Tarek (2020-06-15)."The development of ghost vaccines trials".Expert Review of Vaccines.19(6): 549–562.doi:10.1080/14760584.2020.1777862.ISSN1476-0584.PMID32500816.S2CID219331100.Archivedfrom the original on 2021-04-25.Retrieved2021-04-25.
- ^abcd"Different Types of Vaccines | History of Vaccines".www.historyofvaccines.org.Archivedfrom the original on 2019-01-26.Retrieved2019-06-14.
- ^"Types of Vaccines".coastalcarolinaresearch.com.Archived fromthe originalon 2019-05-03.Retrieved2019-05-03.
- ^Philadelphia, The Children's Hospital of (2014-08-18)."A Look at Each Vaccine: Hepatitis B Vaccine".www.chop.edu.Archivedfrom the original on 2019-05-31.Retrieved2019-06-14.
- ^"HPV Vaccine | Human Papillomavirus | CDC".www.cdc.gov.2019-05-13.Archivedfrom the original on 2019-06-18.Retrieved2019-06-14.
- ^Williamson, E. D.; Eley, S. M.; Griffin, K. F.; Green, M.; Russell, P.; Leary, S. E.; Oyston, P. C.; Easterbrook, T.; Reddin, K. M. (December 1995)."A new improved sub-unit vaccine for plague: the basis of protection".FEMS Immunology and Medical Microbiology.12(3–4): 223–230.doi:10.1111/j.1574-695X.1995.tb00196.x.ISSN0928-8244.PMID8745007.
- ^"Polysaccharide Protein Conjugate Vaccines".www.globalhealthprimer.emory.edu.Archivedfrom the original on 2019-06-23.Retrieved2019-06-14.
- ^abcPollard AJ, Bijker EM (2020-12-22)."A guide to vaccinology: from basic principles to new developments".Nature Reviews Immunology.21(2): 83–100.doi:10.1038/s41577-020-00479-7.ISSN1474-1741.PMC7754704.PMID33353987.
- ^Pol L, Stork M, Ley P (2015-11-11)."Outer membrane vesicles as platform vaccine technology".Biotechnology Journal.10(11): 1689–1706.doi:10.1002/biot.201400395.ISSN1860-7314.PMC4768646.PMID26912077.
- ^Scott (April 2004)."Classifying Vaccines"(PDF).BioProcesses International:14–23.Archived(PDF)from the original on 2013-12-12.Retrieved2014-01-09.
- ^"Vaccine Types".Vaccines.org.Office of Infectious Disease of theUnited States Department of Health and Human Services.Archivedfrom the original on 23 May 2019.Retrieved13 March2021.
- ^"Understanding and Explaining Viral Vector COVID-19 Vaccines".Centers for Disease Control and Prevention.Archivedfrom the original on 2 February 2021.Retrieved13 March2021.
- ^Garde, Damian; Feuerstein, Adam (1 November 2020)."How nanotechnology helps mRNA Covid-19 vaccines work".STAT.Archivedfrom the original on 1 December 2020.Retrieved21 December2020.
- ^CDC (11 February 2020)."COVID-19 and Your Health".Centers for Disease Control and Prevention.Archivedfrom the original on 3 March 2021.Retrieved21 December2020.
- ^Banks, Marcus A. (16 July 2020)."What Are mRNA Vaccines, and Could They Work Against COVID-19?".Smithsonian Magazine.Archivedfrom the original on 21 December 2020.Retrieved21 December2020.
- ^Branswell, Helen (19 December 2020)."FDA grants authorization to Moderna's Covid-19 vaccine".STAT.Archivedfrom the original on 21 December 2020.Retrieved21 December2020.
- ^Cuffari, Benedette (17 March 2021)."What is a DNA Vaccine?".News-Medical.net.Retrieved2024-01-14.
- ^"DNA Vaccines".World Health Organization.Retrieved2024-01-14.
- ^Kim W, Liau LM (January 2010)."Dendritic cell vaccines for brain tumors".Neurosurgery Clinics of North America.21(1): 139–157.doi:10.1016/j.nec.2009.09.005.PMC2810429.PMID19944973.
- ^Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN (June 2014). "Clinical use of dendritic cells for cancer therapy".The Lancet. Oncology.15(7): e257–267.doi:10.1016/S1470-2045(13)70585-0.PMID24872109.
- ^McKenzie, David (26 May 2018)."Fear and failure: How Ebola sparked a global health revolution".CNN.Archivedfrom the original on 26 August 2019.Retrieved26 May2018.
- ^Meri S, Jördens M, Jarva H (December 2008). "Microbial complement inhibitors as vaccines".Vaccine.26(Suppl 8): I113–117.doi:10.1016/j.vaccine.2008.11.058.PMID19388175.
- ^Lowe (2008)."Plasmid DNA as Prophylactic and Therapeutic vaccines for Cancer and Infectious Diseases".Plasmids: Current Research and Future Trends.Caister Academic Press.ISBN978-1-904455-35-6.Archivedfrom the original on 2008-04-11.Retrieved2008-04-15.
- ^Chang, Lee-Jah; Blair, Wade (11 December 2023)."Mimicking nature: Virus-like particles and the next generation of vaccines".AstraZeneca.
- ^Cambridge, University of."'Quartet Nanocage' vaccine found effective against coronaviruses that haven't even emerged yet ".phys.org.Retrieved2024-05-06.
- ^"Monovalent"atDorland's Medical Dictionary
- ^"Polyvalent vaccine".Dorland's Medical Dictionary.2012-03-07. Archived fromthe originalon 2012-03-07.
- ^"Questions And Answers On Monovalent Oral Polio Vaccine Type 1 (mOPV1)'Issued Jointly By WHO and UNICEF'".Pediatric Oncall.2(8). 3. What advantages does mOPV1 have over trivalent oral polio vaccine (tOPV)?. 2005-01-08. Archived fromthe originalon 2012-02-29.
- ^Gizurarson, Sveinbj??rn (1998)."Clinically Relevant Vaccine-Vaccine Interactions: A Guide for Practitioners".BioDrugs.9(6): 443–453.doi:10.2165/00063030-199809060-00002.PMID18020577.
- ^Sutter RW, Cochi SL, Melnick JL (1999). "Live attenuated polio vaccines". In Plotkin SA, Orenstein WA (eds.).Vaccines.Philadelphia: W. B. Saunders. pp. 364–408.
- ^Kanesa-thasan N, Sun W, Kim-Ahn G, Van Albert S, Putnak JR, King A, Raengsakulsrach B, Christ-Schmidt H, Gilson K, Zahradnik JM, Vaughn DW, Innis BL, Saluzzo JF, Hoke CH (April 2001). "Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers".Vaccine.19(23–24): 3179–3188.CiteSeerX10.1.1.559.8311.doi:10.1016/S0264-410X(01)00020-2.PMID11312014.
- ^Engler, Renata J. M.; Greenwood, John T.; Pittman, Phillip R.; Grabenstein, John D. (2006-08-01)."Immunization to Protect the US Armed Forces: Heritage, Current Practice, and Prospects".Epidemiologic Reviews.28(1): 3–26.doi:10.1093/epirev/mxj003.ISSN0193-936X.PMID16763072.
- ^Sox, Harold C.; Liverman, Catharyn T.; Fulco, Carolyn E.; War, Institute of Medicine (US) Committee on Health Effects Associated with Exposures During the Gulf (2000).Vaccines.National Academies Press (US).Archivedfrom the original on 2021-11-16.Retrieved2019-05-03.
- ^"Institute for Vaccine Safety – Thimerosal Table".Archivedfrom the original on 2005-12-10.
- ^Wharton, Melinda E.; National Vaccine Advisory committee"U.S.A. national vaccine plan"Archived2016-05-04 at theWayback Machine
- ^"Measurements of Non-gaseous air pollutants > Metals".npl.co.uk.National Physics Laboratory. Archived fromthe originalon 29 September 2007.Retrieved28 June2020.
- ^"Thimerosal in vaccines".Center for Biologics Evaluation and Research, U.S. Food and Drug Administration. 2007-09-06.Archivedfrom the original on 2013-01-06.Retrieved2007-10-01.
- ^Bigham M, Copes R (2005). "Thiomersal in vaccines: balancing the risk of adverse effects with the risk of vaccine-preventable disease".Drug Safety.28(2): 89–101.doi:10.2165/00002018-200528020-00001.PMID15691220.S2CID11570020.
- ^Offit PA(September 2007)."Thimerosal and vaccines – a cautionary tale".The New England Journal of Medicine.357(13): 1278–1279.doi:10.1056/NEJMp078187.PMID17898096.S2CID36318722.
- ^"Another study, this one of 657k kids, finds MMR vaccine doesn't cause autism".National Post.2019-03-05.Retrieved2019-03-13.
- ^Hoffman J (2019-03-05)."One More Time, With Big Data: Measles Vaccine Doesn't Cause Autism".The New York Times.ISSN0362-4331.Archivedfrom the original on 2019-03-12.Retrieved2019-03-13.
- ^CDC (2018-07-12)."Ingredients of Vaccines – Fact Sheet".Archivedfrom the original on December 17, 2009.RetrievedDecember 20,2009.
- ^The mercury levels in the table, unless otherwise indicated, are taken fromMercury Levels in Commercial Fish and Shellfish (1990–2010)Archived2015-05-03 at theWayback MachineU.S. Food and Drug Administration. Accessed 8January 2012.
- ^abCenters for Disease Control and Prevention (12 November 2020),U.S. Vaccine Names,archivedfrom the original on 2021-08-21,retrieved2021-08-21.
- ^abCenters for Disease Control and Prevention (2018-08-07),Tetanus (Lockjaw) Vaccination,archivedfrom the original on 2016-05-16,retrieved2016-05-21.
- ^Centers for Disease Control and Prevention (2018-02-02),Vaccine Acronyms and Abbreviations [Abbreviations used on U.S. immunization records],archivedfrom the original on 2017-06-02,retrieved2017-05-22.
- ^abcdefghi"Principles and considerations for adding a vaccine to a national immunization programme"(PDF).World Health Organization. 1 April 2014.Archived(PDF)from the original on 29 September 2020.Retrieved17 August2020.
- ^Bok, Karin; Sitar, Sandra; Graham, Barney S.;Mascola, John R.(August 2021)."Accelerated COVID-19 vaccine development: milestones, lessons, and prospects".Immunity.54(8): 1636–1651.doi:10.1016/j.immuni.2021.07.017.PMC8328682.PMID34348117.
- ^abcdWijnans, Leonoor; Voordouw, Bettie (11 December 2015)."A review of the changes to the licensing of influenza vaccines in Europe".Influenza and Other Respiratory Viruses.10(1): 2–8.doi:10.1111/irv.12351.ISSN1750-2640.PMC4687503.PMID26439108.
- ^Offit, Paul A. (2020)."Making vaccines: Licensure, recommendations and requirements".Children's Hospital of Philadelphia.Archivedfrom the original on 8 September 2020.Retrieved20 August2020.
- ^abToner E, Barnill A, Krubiner C, Bernstein J, Privor-Dumm L, Watson M, et al. (2020).Interim Framework for COVID-19 Vaccine Allocation and Distribution in the United States(PDF)(Report). Baltimore, MD: Johns Hopkins Center for Health Security.Archived(PDF)from the original on 22 August 2020.Retrieved24 August2020.
- ^Dooling K, Marin M, Wallace M, McClung N, Chamberland M, Lee GM, et al. (December 2020)."The Advisory Committee on Immunization Practices' Updated Interim Recommendation for Allocation of COVID-19 Vaccine – United States, December 2020".MMWR. Morbidity and Mortality Weekly Report.69(5152): 1657–1660.doi:10.15585/mmwr.mm695152e2.PMC9191902.PMID33382671.
- ^abcde"Vaccine product approval process".U.S.Food and Drug Administration(FDA). 30 January 2020. Archived fromthe originalon 27 September 2020.Retrieved17 August2020.
- ^"home".Cdsco.gov.in. 2021-04-15.Archivedfrom the original on 2022-01-04.Retrieved2022-01-10.
- ^Steffen, Christoph A.; Henaff, Louise; et al. (8 April 2021)."Evidence-informed vaccination decision-making in countries: Progress, challenges and opportunities".Vaccine.39(15). Elsevier: 2146–2152.doi:10.1016/j.vaccine.2021.02.055.PMID33712350.
- ^"ACIP Vaccine Recommendations Home Page".CDC. 2013-11-15.Archivedfrom the original on 2013-12-31.Retrieved2014-01-10.
- ^"Vaccine Status Table".Red Book Online.American Academy of Pediatrics. April 26, 2011.Archivedfrom the original on December 27, 2013.RetrievedJanuary 9,2013.
- ^"HPV Vaccine Safety".Centers for Disease Control and Prevention (CDC). 2013-12-20.Archivedfrom the original on 2009-11-10.Retrieved2014-01-10.
- ^"HPV vaccine in the clear".NHS choices.2009-10-02.Archivedfrom the original on 2014-01-10.Retrieved2014-01-10.
- ^"Zostavax EPAR".European Medicines Agency(EMA).29 July 2021.Archivedfrom the original on 5 August 2020.Retrieved1 September2021.
- ^Dooling, Kathleen (2021-08-13)."The Advisory Committee on Immunization Practices' Updated Interim Recommendation for Allocation of COVID-19 Vaccine – United States, December 2020"(PDF).CDC the Advisory Committee on Immunization Practices.69(5152): 1657–1660.PMC9191902.PMID33382671.Archived(PDF)from the original on 2021-08-19.Retrieved2021-08-17.
- ^Hunziker, Patrick (2021-07-24)."Personalized-dose Covid-19 vaccination in a wave of virus Variants of Concern: Trading individual efficacy for societal benefit".Precision Nanomedicine.4(3): 805–820.doi:10.33218/001c.26101.ISSN2639-9431.Archivedfrom the original on 2021-10-09.Retrieved2021-08-17.
- ^Goodman JL (2005-05-04)."Statement by Jesse L. Goodman, M.D., M.P.H. Director Center for Biologics, Evaluation and Research Food and Drug Administration U.S. Department of Health and Human Services on US Influenza Vaccine Supply and Preparations for the Upcoming Influenza Season before Subcommittee on Oversight and Investigations Committee on Energy and Commerce United States House of Representatives".Archivedfrom the original on 2008-09-21.Retrieved2008-06-15.
- ^Olesen OF, Lonnroth A, Mulligan B (January 2009)."Human vaccine research in the European Union".Vaccine.27(5): 640–645.doi:10.1016/j.vaccine.2008.11.064.PMC7115654.PMID19059446.
- ^Jit M, Newall AT, Beutels P (April 2013)."Key issues for estimating the impact and cost-effectiveness of seasonal influenza vaccination strategies".Human Vaccines & Immunotherapeutics.9(4): 834–840.doi:10.4161/hv.23637.PMC3903903.PMID23357859.
- ^Newall AT, Reyes JF, Wood JG, McIntyre P, Menzies R, Beutels P (February 2014). "Economic evaluations of implemented vaccination programmes: key methodological challenges in retrospective analyses".Vaccine.32(7): 759–765.doi:10.1016/j.vaccine.2013.11.067.PMID24295806.
- ^Roser, Max; Vanderslott, Samantha (2013-05-10)."Vaccination".Our World in Data.Archivedfrom the original on 2020-09-01.Retrieved2019-05-03.
- ^"Increasing Access to Vaccines Through Technology Transfer and Local Production"(PDF).World Health Organization. 2011.Archived(PDF)from the original on 2015-11-23.
- ^Christy Somos (7 May 2021)."Everything you need to know about the WTO's COVID-19 vaccine patent proposal".CTV News.Archivedfrom the original on 23 May 2021.Retrieved23 May2021.
- ^abGomez, Phillip L.; Robinson, James M.; Rogalewicz, James (2008)."Chapter 4: Vaccine Manufacturing".In Plotkin, Stanley A.; Orenstein, Walter A.; Offit, Paul A. (eds.).Vaccines(5th ed.). New York: Saunders Elsevier. pp. 45–58.ISBN978-1-4377-2158-4.Archivedfrom the original on April 18, 2023.RetrievedMarch 26,2021.
- ^abcPlotkin, Stanley; Robinson, James M.; Cunningham, Gerard; Iqbal, Robyn; Larsen, Shannon (24 July 2017)."The complexity and cost of vaccine manufacturing – An overview".Vaccine.35(33): 4064–4071.doi:10.1016/j.vaccine.2017.06.003.PMC5518734.PMID28647170.
- ^"Three ways to make a vaccine"(infographic).Archivedfrom the original on 2015-12-23.Retrieved2015-08-05,inStein, Rob (24 November 2009)."Vaccine system remains antiquated".The Washington Post.Archivedfrom the original on 19 October 2017.
- ^abMuzumdar JM, Cline RR (2009)."Vaccine supply, demand, and policy: a primer".Journal of the American Pharmacists Association.49(4): e87–99.doi:10.1331/JAPhA.2009.09007.PMC7185851.PMID19589753.
- ^ab"Components of a vaccine".Archivedfrom the original on 2017-06-13.
- ^abBae K, Choi J, Jang Y, Ahn S, Hur B (April 2009)."Innovative vaccine production technologies: the evolution and value of vaccine production technologies".Archives of Pharmacal Research.32(4): 465–480.doi:10.1007/s12272-009-1400-1.PMID19407962.S2CID9066150.
- ^"Vaccine Taskforce Aims"(PDF).assets.publishing.service.gov.uk.6 April 2020.Archived(PDF)from the original on 2020-07-26.Retrieved2020-07-26.
- ^Pagliusi, Sonia; Jarrett, Stephen; Hayman, Benoit; Kreysa, Ulrike; Prasad, Sai D.; Reers, Martin; Hong Thai, Pham; Wu, Ke; Zhang, Youn Tao; Baek, Yeong Ok; Kumar, Anand (July 2020)."Emerging manufacturers engagements in the COVID −19 vaccine research, development and supply".Vaccine.38(34): 5418–5423.doi:10.1016/j.vaccine.2020.06.022.PMC7287474.PMID32600908.
- ^Miller, Joe; Kuchler, Hannah (2020-04-28)."Drugmakers race to scale up vaccine capacity".www.ft.com.Archived fromthe originalon 2022-12-10.Retrieved2020-07-26.
- ^abcdefPlotkin, Stanley A.; Orenstein, Walter A.; Offit, Paul A.; Edwards, Kathryn M. (2017).Vaccines.Elsevier.ISBN978-0-323-39301-0.
- ^Morein B, Hu KF, Abusugra I (June 2004). "Current status and potential application of ISCOMs in veterinary medicine".Advanced Drug Delivery Reviews.56(10): 1367–1382.doi:10.1016/j.addr.2004.02.004.PMID15191787.
- ^American Medicine.American-Medicine Publishing Company. 1926.
- ^South African Institute for Medical Research (1929).Annual report [Jaarverslag].South African Institute for Medical Research – Suid-Afrikaanse Instituut vir Mediese Navorsing.
- ^Khan FA (2011-09-20).Biotechnology Fundamentals.CRC Press. p. 270.ISBN978-1-4398-2009-4.
- ^Giudice EL, Campbell JD (April 2006). "Needle-free vaccine delivery".Advanced Drug Delivery Reviews.58(1): 68–89.doi:10.1016/j.addr.2005.12.003.PMID16564111.
- ^WHOto trial Nanopatch needle-free delivery system|ABC News,16 Sep 2014|"Needle-free polio vaccine a 'game-changer'".ABC News.2014-09-16.Archivedfrom the original on 2015-04-02.Retrieved2015-09-15.
- ^"Australian scientists develop 'needle-free' vaccination".The Sydney Morning Herald.18 August 2013.Archivedfrom the original on 25 September 2015.
- ^"Vaxxas raises $25m to take Brisbane's Nanopatch global".Business Review Weekly.2015-02-10. Archived fromthe originalon 2015-03-16.Retrieved2015-03-05.
- ^"Australian scientists develop 'needle-free' vaccination".The Hindu.Chennai, India. 28 September 2011.Archivedfrom the original on 1 January 2014.
- ^"Needle-free nanopatch vaccine delivery system".News Medical. 3 August 2011.Archivedfrom the original on 11 May 2012.
- ^Patel JR, Heldens JG (March 2009)."Immunoprophylaxis against important virus disease of horses, farm animals and birds".Vaccine.27(12): 1797–1810.doi:10.1016/j.vaccine.2008.12.063.PMC7130586.PMID19402200.
- ^abBerkelman RL (August 2003)."Human illness associated with use of veterinary vaccines".Clinical Infectious Diseases.37(3): 407–414.doi:10.1086/375595.PMID12884166.
- ^van Oirschot JT, Rziha HJ, Moonen PJ, Pol JM, van Zaane D (June 1986)."Differentiation of serum antibodies from pigs vaccinated or infected with Aujeszky's disease virus by a competitive enzyme immunoassay".The Journal of General Virology.67 (Pt 6) (6): 1179–1182.doi:10.1099/0022-1317-67-6-1179.PMID3011974.
- ^abvan Oirschot JT (August 1999). "Diva vaccines that reduce virus transmission".Journal of Biotechnology.73(2–3): 195–205.doi:10.1016/S0168-1656(99)00121-2.PMID10486928.
- ^van Oirschot JT, Gielkens AL, Moormann RJ, Berns AJ (June 1990). "Marker vaccines, virus protein-specific antibody assays and the control of Aujeszky's disease".Veterinary Microbiology.23(1–4): 85–101.doi:10.1016/0378-1135(90)90139-M.PMID2169682.
- ^Kaashoek MJ, Moerman A, Madić J, Rijsewijk FA, Quak J, Gielkens AL, van Oirschot JT (April 1994). "A conventionally attenuated glycoprotein E-negative strain of bovine herpesvirus type 1 is an efficacious and safe vaccine".Vaccine.12(5): 439–444.doi:10.1016/0264-410X(94)90122-8.PMID8023552.
- ^Hulst MM, Westra DF, Wensvoort G, Moormann RJ (September 1993)."Glycoprotein E1 of hog cholera virus expressed in insect cells protects swine from hog cholera".Journal of Virology.67(9): 5435–5442.doi:10.1128/JVI.67.9.5435-5442.1993.PMC237945.PMID8350404.
- ^Capua I, Terregino C, Cattoli G, Mutinelli F, Rodriguez JF (February 2003)."Development of a DIVA (Differentiating Infected from Vaccinated Animals) strategy using a vaccine containing a heterologous neuraminidase for the control of avian influenza".Avian Pathology.32(1): 47–55.doi:10.1080/0307945021000070714.PMID12745380.S2CID22827454.
- ^Maas A, Meens J, Baltes N, Hennig-Pauka I, Gerlach GF (November 2006). "Development of a DIVA subunit vaccine against Actinobacillus pleuropneumoniae infection".Vaccine.24(49–50): 7226–7237.doi:10.1016/j.vaccine.2006.06.047.PMID17027123.
- ^Leyman B, Boyen F, Van Parys A, Verbrugghe E, Haesebrouck F, Pasmans F (May 2011)."Salmonella Typhimurium LPS mutations for use in vaccines allowing differentiation of infected and vaccinated pigs".Vaccine.29(20): 3679–3685.doi:10.1016/j.vaccine.2011.03.004.hdl:1854/LU-1201519.PMID21419163.Archivedfrom the original on 2017-10-28.
- ^Needham, Joseph (2000).Science and Civilisation in China: Volume 6, Biology and Biological Technology, Part 6, Medicine.Cambridge University Press. p. 154.ISBN978-0-521-63262-1.
- ^abWilliams G (2010).Angel of Death.Basingstoke: Palgrave Macmillan.ISBN978-0-230-27471-6.
- ^Silverstein, Arthur M. (2009).A History of Immunology(2nd ed.). Academic Press. p. 293.ISBN978-0-08-091946-1.
- ^Voltaire (1742)."Letter XI".Letters on the English.Archivedfrom the original on 2018-10-16.Retrieved2023-07-26.
- ^Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I.D. (1988).Smallpox and its Eradication.Geneva: World Health Organization.ISBN92-4-156110-6.
- ^abBaxby, Derrick (1984)."A Death from Inoculated Smallpox in the English Royal Family".Med Hist.28(3): 303–307.doi:10.1017/s0025727300035961.PMC1139449.PMID6390027.
- ^abcStern AM, Markel H (2005)."The history of vaccines and immunization: familiar patterns, unew challenges".Health Affairs.24(3): 611–621.doi:10.1377/hlthaff.24.3.611.PMID15886151.
- ^Dunn PM (January 1996)."Dr Edward Jenner (1749-1823) of Berkeley, and vaccination against smallpox"(PDF).Archives of Disease in Childhood: Fetal and Neonatal Edition.74(1): F77–78.doi:10.1136/fn.74.1.F77.PMC2528332.PMID8653442.Archived fromthe original(PDF)on 2011-07-08.
- ^Exhibition tells story of Spanish children used as vaccine fridges in 1803Archived2022-08-30 at theWayback MachineThe Guardian, 2021
- ^Van Sant JE (2008). "The Vaccinators: Smallpox, Medical Knowledge, and the 'Opening' of Japan".J Hist Med Allied Sci.63(2): 276–279.doi:10.1093/jhmas/jrn014.
- ^Didgeon JA (May 1963)."Development of Smallpox Vaccine in England in the Eighteenth and Nineteenth Centuries".British Medical Journal.1(5342): 1367–1372.doi:10.1136/bmj.1.5342.1367.PMC2124036.PMID20789814.
- ^Louten, Jennifer (2016).Essential Human Virology.Academic Press. pp. 134–135.ISBN978-0-12-801171-3.
- ^Baarda BI, Sikora AE (2015)."Proteomics of Neisseria gonorrhoeae: the treasure hunt for countermeasures against an old disease".Frontiers in Microbiology.6:1190.doi:10.3389/fmicb.2015.01190.PMC4620152.PMID26579097;
- ^abcAlarcon JB, Waine GW, McManus DP (1999). "DNA Vaccines: Technology and Application as Anti-parasite and Anti-microbial Agents".Advances in Parasitology Volume 42.Vol. 42. pp. 343–410.doi:10.1016/S0065-308X(08)60152-9.ISBN978-0-12-031742-4.PMID10050276.
- ^Robinson HL, Pertmer TM (2000).DNA vaccines for viral infections: basic studies and applications.Advances in Virus Research. Vol. 55. pp. 1–74.doi:10.1016/S0065-3527(00)55001-5.ISBN978-0-12-039855-3.PMID11050940.
- ^Naftalis, Kramer Levin; Royzman, Frankel LLP-Irena; Pineda, ré (30 November 2020)."Third-Generation Vaccines Take Center Stage in Battle Against COVID-19 | Lexology".www.lexology.com.Archivedfrom the original on 30 January 2021.Retrieved24 January2021.
- ^Regalado, Antonio."The U.S. government has begun testing its first Zika vaccine in humans".Archivedfrom the original on 2016-08-21.Retrieved2016-08-06.
- ^Chen Y, Wang S, Lu S (February 2014)."DNA Immunization for HIV Vaccine Development".Vaccines.2(1): 138–159.doi:10.3390/vaccines2010138.PMC4494200.PMID26344472.
- ^"Katalin Karikó and Drew Weissman Awarded Horwitz Prize for Pioneering Research on COVID-19 Vaccines".Columbia University Irving Medical Center.2021-08-12.Archivedfrom the original on 2021-08-16.Retrieved2021-09-07.
- ^Staff (28 March 2013)."Safer vaccine created without virus".The Japan Times.Agence France-Presse – Jiji Press.Archivedfrom the original on 30 March 2013.Retrieved2013-03-28.
- ^Spohn G, Bachmann MF (February 2008). "Exploiting viral properties for the rational design of modern vaccines".Expert Review of Vaccines.7(1): 43–54.doi:10.1586/14760584.7.1.43.PMID18251693.S2CID40130001.
- ^Samuelsson O, Herlitz H (March 2008). "Vaccination against high blood pressure: a new strategy".Lancet.371(9615): 788–789.doi:10.1016/S0140-6736(08)60355-4.PMID18328909.S2CID38323966.
- ^Poland GA, Jacobson RM, Ovsyannikova IG (May 2009)."Trends affecting the future of vaccine development and delivery: the role of demographics, regulatory science, the anti-vaccine movement, and vaccinomics".Vaccine.27(25–26): 3240–3244.doi:10.1016/j.vaccine.2009.01.069.PMC2693340.PMID19200833.
- ^Sala F, Manuela Rigano M, Barbante A, Basso B, Walmsley AM, Castiglione S (January 2003). "Vaccine antigen production in transgenic plants: strategies, gene constructs and perspectives".Vaccine.21(7–8): 803–808.doi:10.1016/s0264-410x(02)00603-5.PMID12531364.
- ^Kumar GB, Ganapathi TR, Revathi CJ, Srinivas L, Bapat VA (October 2005). "Expression of hepatitis B surface antigen in transgenic banana plants".Planta.222(3): 484–493.Bibcode:2005Plant.222..484K.doi:10.1007/s00425-005-1556-y.PMID15918027.S2CID23987319.
- ^abLeonhardt, David (March 11, 2024)."The Fourth Anniversary of the Covid Pandemic".The New York Times.Archivedfrom the original on March 11, 2024."Data excludes Alaska. Sources: C.D.C. Wonder; Edison Research. (Chart) By The New York Times. Source credits chart to Ashley Wu.
- ^The Lancet Child & Adolescent Health (2019)."Vaccine hesitancy: a generation at risk".The Lancet.3(5): 281.doi:10.1016/S2352-4642(19)30092-6.PMID30981382.S2CID115201206.
- ^Smith, MJ (November 2015). "Promoting Vaccine Confidence".Infectious Disease Clinics of North America(Review).29(4): 759–69.doi:10.1016/j.idc.2015.07.004.PMID26337737.
- ^Larson, HJ; Jarrett, C; Eckersberger, E; Smith, DM; Paterson, P (April 2014). "Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012".Vaccine.32(19): 2150–59.doi:10.1016/j.vaccine.2014.01.081.PMID24598724.
- ^Cataldi, Jessica; O'Leary, Sean (2021)."Parental vaccine hesitancy: scope, causes, and potential responses".Current Opinion in Infectious Diseases.34(5): 519–526.doi:10.1097/QCO.0000000000000774.PMID34524202.S2CID237437018.Archivedfrom the original on 2023-12-24.Retrieved2022-06-24.
- ^"Communicating science-based messages on vaccines".Bulletin of the World Health Organization.95(10): 670–71. October 2017.doi:10.2471/BLT.17.021017.PMC5689193.PMID29147039.
- ^"Why do some people oppose vaccination?".Vox.Archivedfrom the original on 2019-09-21.Retrieved2018-11-26.
- ^Ceccarelli L."Defending science: How the art of rhetoric can help".The Conversation.Archivedfrom the original on 2019-11-05.Retrieved2018-11-26.
- ^U.S. Department of Health and Human Services."Vaccines.gov".Vaccines.gov.Archivedfrom the original on 2019-03-13.Retrieved2018-08-05.
- ^"Frequently Asked Questions (FAQ)".Boston Children's Hospital.Archived fromthe originalon October 17, 2013.RetrievedFebruary 11,2014.
- ^Phadke VK, Bednarczyk RA, Salmon DA, Omer SB (March 2016)."Association Between Vaccine Refusal and Vaccine Preventable Diseases in the United States: A Review of Measles and Pertussis".JAMA.315(11): 1149–58.doi:10.1001/jama.2016.1353.PMC5007135.PMID26978210.
- ^Wolfe R, Sharp L (2002)."Anti-vaccinationists past and present".BMJ.325(7361): 430–2.doi:10.1136/bmj.325.7361.430.PMC1123944.PMID12193361.Archivedfrom the original on 2006-08-25.Retrieved2008-01-14.
- ^Poland GA, Jacobson RM (January 2011). "The age-old struggle against the antivaccinationists".The New England Journal of Medicine.364(2): 97–99.doi:10.1056/NEJMp1010594.PMID21226573.S2CID39229852.
- ^Wallace A (2009-10-19)."An epidemic of fear: how panicked parents skipping shots endangers us all".Wired.Archivedfrom the original on 2013-12-25.Retrieved2009-10-21.
- ^Poland GA, Jacobson RM (March 2001). "Understanding those who do not understand: a brief review of the anti-vaccine movement".Vaccine.19(17–19): 2440–45.doi:10.1016/S0264-410X(00)00469-2.PMID11257375.S2CID1978650.
- ^"Ten threats to global health in 2019".Who.int.Archived fromthe originalon 2019-06-27.Retrieved2020-12-09.
- ^PM, Aristos Georgiou (2019-01-15)."The anti-vax movement has been listed by WHO as one of its top 10 health threats for 2019".Archivedfrom the original on 2019-11-22.Retrieved2019-01-16.
Further reading
- Hall E, Wodi AP, Hamborsky J, Morelli V, Schillie S, eds. (2021).Epidemiology and Prevention of Vaccine-Preventable Diseases(14th ed.). Washington D.C.: U.S.Centers for Disease Control and Prevention(CDC).
External links
| External videos | |
|---|---|
| Modern Vaccine and Adjuvant Production and Characterization,Genetic Engineering & Biotechnology News |
- Vaccines and AntiseraatCurlie
- WHO Vaccine preventable diseases and immunization
- World Health Organization position papers on vaccines
- The History of Vaccines,from theCollege of Physicians of Philadelphia
- This website was highlighted byGenetic Engineering & Biotechnology Newsin its "Best of the Web" section in January 2015. See:"The History of Vaccines". Best of the Web.Genetic Engineering & Biotechnology News.Vol. 35, no. 2. 15 January 2015. p. 38.