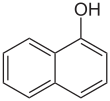
1-Naphthol,orα-naphthol,is anorganic compoundwith the formulaC10H7OH.It is afluorescentwhite solid. 1-Naphthol differs from itsisomer2-naphtholby the location of thehydroxyl groupon thenaphthalenering. The naphthols are naphthalene homologues ofphenol.Both isomers are soluble in simpleorganic solvents.They are precursors to a variety of useful compounds.[2]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Naphthalen-1-ol | |||
| Other names
1-Hydroxynaphthalene; 1-Naphthalenol; α-Naphthol
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1817321 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.791 | ||
| EC Number |
| ||
| 69192 | |||
| KEGG | |||
PubChemCID
|
|||
| UNII | |||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H8O | |||
| Molar mass | 144.17 g/mol | ||
| Appearance | Colorless or white solid | ||
| Density | 1.10 g/cm3 | ||
| Melting point | 95 to 96 °C (203 to 205 °F; 368 to 369 K) | ||
| Boiling point | 278 to 280 °C (532 to 536 °F; 551 to 553 K) | ||
| -98.2·10−6cm3/mol | |||
| Hazards | |||
| GHSlabelling:[1] | |||
   
| |||
| Danger | |||
| H302,H311,H312,H315,H317,H318,H335,H410,H412 | |||
| P261,P262,P264,P264+P265,P270,P271,P272,P273,P280,P301+P317,P302+P352,P304+P340,P305+P354+P338,P316,P317,P319,P321,P330,P332+P317,P333+P317,P361+P364,P362+P364,P391,P403+P233,P405,P501 | |||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
Production
edit1-Naphthol is prepared by two main routes.[2]In one method, naphthalene is nitrated to give 1-nitronaphthalene, which is hydrogenated to the amine followed by hydrolysis:
- C10H8+ HNO3→ C10H7NO2+ H2O
- C10H7NO2+ 3H2→ C10H7NH2+ 2H2O
- C10H7NH2+ H2O → C10H7OH + NH3
Alternatively, naphthalene is hydrogenated totetralin,which is oxidized to1-tetralone,which undergoesdehydrogenation.
Reactions
editSome reactions of 1-naphthol are explicable with reference to its tautomerism, which produces a small amount of the keto tautomer.[citation needed]
One consequence of this tautomerism is theBucherer reaction,the ammonolysis of 1-naphthol to give1-aminonaphthalene.
1-Naphthol biodegrades via formation of1-naphthol-3,4-oxide,which converts to1,4-naphthoquinone.[3]
The 4-position of 1-naphthol is susceptible to electrophilic attack. This regioselective reaction is exploited in the preparation of diazo dyes, which are form usingdiazonium salts.Reduction of the diazo derivatives gives 4-amino-1-naphthol.[4][5]
Partial reduction of 1-naphthol gives the tetrahydro derivative, leaving intact the phenol ring.[6]Full hydrogenation is catalyzed by rhodium.[7]
Applications and occurrence
edit1-Naphthol is a precursor to a variety of insecticides includingcarbaryland pharmaceuticals includingnadolol[8][9]as well as for theantidepressantsertraline[10]and theanti-protozoantherapeuticatovaquone.[11]It undergoesazo couplingto give variousazo dyes,but these are generally less useful than those derived from 2-naphthol.[2][12]
1-Naphthol is a metabolite of the insecticidecarbarylandnaphthalene.Along withTCPy,it has been shown to decrease testosterone levels in adult men.[13]
Other uses
edit1-Naphthol is used in each of the following chemical tests, which predate the use of spectroscopic and chromatographic methods:
- Molisch's testgives a red- or purple-colored compound to indicate the presence ofcarbohydrate.
- rapid furfural testturns purple quickly (<30s) iffructoseis present, distinguishing it from glucose.
- Sakaguchi testturns red to indicate the presence ofargininein proteins.
- Voges–Proskauer testchanges color from yellow to red to indicate thatglucoseis being broken down intoacetoinwhich is used by bacteria for external energy storage.
Safety
edit1-Naphthol has been described as "moderately toxic".[2]
References
edit- ^"1-Naphthol".pubchem.ncbi.nlm.nih.gov.
- ^abcdBooth, Gerald (2005). "Naphthalene Derivatives".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a17_009.ISBN978-3-527-30673-2..full-text PDF
- ^Yoshito Kumagai; Yasuhiro Shinkai; Takashi Miura;Arthur K. Cho(2011). "The Chemical Biology of Naphthoquinones and Its Environmental Implications".Annual Review of Pharmacology and Toxicology.52:221–47.doi:10.1146/annurev-pharmtox-010611-134517.PMID21942631.
- ^J. B. Conant; R. E. Lutz; B. B. Corson (1923). "1,4-Aminonaphthol Hydrochloride".Organic Syntheses.3:7.doi:10.15227/orgsyn.003.0007.
- ^Louis F. Fieser (1937). "1,2-Aminonaphthol Hydrochloride".Organic Syntheses.17:9.doi:10.15227/orgsyn.017.0009.
- ^C. David Gutsche; Hugo H. Peter (1957). "Ar-Tetrahydro-a-Naphthol".Organic Syntheses.37:80.doi:10.15227/orgsyn.037.0080.
- ^A. I. Meyers; W. N. Beverung; R. Gault (1971). "Hydrogenation of Aromatic Nuclei: 1-Decalol".Organic Syntheses.51:103.doi:10.15227/orgsyn.051.0103.
- ^M.E. Condon; et al. (1978). "Nondepressant β-adrenergic blocking agents. 1. Substituted 3-amino-1-(5,6,7,8-tetrahydro-1-naphthoxy)-2-propanols".Journal of Medicinal Chemistry(in German).21(9):913–922.doi:10.1021/jm00207a014.PMID31485.
- ^DE 2258995,F.R. Hauck, C.M. Cimarusti, V.L. Narayan, "2,3-cis-1,2,3,4-Tetrahydro-5[2-hydroxy-3-(tert.-butylamino)-propoxy]-2,3-naphthalindiol", published 1973-06-07, assigned to E.R. Squibb & Sons, Inc.
- ^K. Vukics; T. Fodor; J. Fischer; I. Fellevári; S. Lévai (2002), "Improved industrial synthesis of antidepressant Sertraline",Org. Process Res. Dev.(in German), vol. 6, no. 1, pp.82–85,doi:10.1021/op0100549
- ^B.N. Roy; G.P. Singh; P.S. Lathi; M.K. Agarwal (2013)."A novel process for synthesis of Atovaquone"(PDF).Indian J. Chem.(in German).52B:1299–1312. Archived fromthe original(PDF)on 30 May 2022.
- ^C. Kaiser; T. Jen; E. Garvey; W.D. Bowen; D.F. Colella; J.R. Wardell Jr. (1977). "Adrenergic agents. 4. Substituted phenoxypropanolamine derivatives as potential β-adrenergic agonists".Journal of Medicinal Chemistry(in German).20(5):687–689.doi:10.1021/jm00215a014.PMID16136.
- ^Meeker, John D.; Ryan, Louise; Barr, Dana B.; Hauser, Russ (January 2006)."Exposure to Nonpersistent Insecticides and Male Reproductive Hormones".Epidemiology.17(1):61–68.doi:10.1097/01.ede.0000190602.14691.70.PMID16357596.S2CID24829926.
External links
edit- NIST Chemistry WebBook 1-Naphthalenol
- .Encyclopædia Britannica.Vol. 19 (11th ed.). 1911. pp.168–169.