Aromatic compoundsorarenesareorganic compounds"with a chemistry typified bybenzene"and" cyclically conjugated. "[1] The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfyingHückel's Rule. Aromatic compounds have the following general properties:
- Typically unreactive
- Often non polar and hydrophobic
- High carbon-hydrogen ratio
- Burn with a strong sooty yellow flame, due to high C:H ratio
- Undergoelectrophilic substitution reactionsandnucleophilic aromatic substitutions[2]
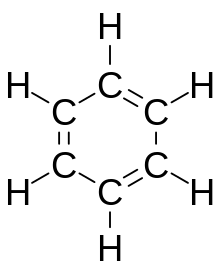
Arenes are typically split into two categories - benzoids, that contain a benzene derivative and follow the benzene ring model, and non-benzoids that contain other aromatic cyclic derivatives. Aromatic compounds are commonly used in organic synthesis and are involved in many reaction types, following both additions and removals, as well as saturation and dearomatization.
Heteroarenes
editHeteroarenesare aromatic compounds, where at least onemethineorvinylene(-C= or -CH=CH-) group is replaced by aheteroatom:oxygen,nitrogen,orsulfur.[3]Examples of non-benzene compounds with aromatic properties arefuran,a heterocyclic compound with a five-membered ring that includes a single oxygen atom, andpyridine,a heterocyclic compound with a six-membered ring containing one nitrogen atom. Hydrocarbons without an aromatic ring are calledaliphatic.Approximately half of compounds known in 2000 are described as aromatic to some extent.[4]
Applications
editAromatic compounds are pervasive in nature and industry. Key industrial aromatic hydrocarbons are benzene,toluene,xylenecalled BTX. Many biomolecules have phenyl groups including the so-calledaromatic amino acids.
Benzene ring model
editBenzene,C6H6,is the least complex aromatic hydrocarbon, and it was the first one defined as such.[6]Its bonding nature was first recognized independently byJoseph LoschmidtandAugust Kekuléin the 19th century.[6]Each carbon atom in the hexagonal cycle has four electrons to share. One electron forms a sigma bond with the hydrogen atom, and one is used in covalently bonding to each of the two neighboring carbons. This leaves six electrons, shared equally around the ring in delocalized pi molecular orbitals the size of the ring itself.[5]This represents the equivalent nature of the six carbon-carbon bonds all ofbond order1.5. This equivalency can also explained byresonance forms.[5]The electrons are visualized as floating above and below the ring, with the electromagnetic fields they generate acting to keep the ring flat.[5]
The circle symbol for aromaticity was introduced bySir Robert Robinsonand his student James Armit in 1925 and popularized starting in 1959 by the Morrison & Boyd textbook on organic chemistry.[7]The proper use of the symbol is debated: some publications use it toanycyclic π system, while others use it only for those π systems that obeyHückel's rule.Some argue that, in order to stay in line with Robinson's originally intended proposal, the use of the circle symbol should be limited to monocyclic 6 π-electron systems.[8]In this way the circle symbol for a six-center six-electron bond can be compared to the Y symbol for athree-center two-electron bond.[8]
Benzene and derivatives of benzene
editBenzene derivatives have from one to sixsubstituentsattached to the central benzene core.[2]Examples of benzene compounds with just one substituent arephenol,which carries ahydroxylgroup, andtoluenewith amethylgroup. When there is more than one substituent present on the ring, their spatial relationship becomes important for which thearene substitution patternsortho,meta,andparaare devised.[9]When reacting to form more complex benzene derivatives, the substituents on a benzene ring can be described as eitheractivatedordeactivated,which are electron donating and electron withdrawing respectively.[9]Activators are known as ortho-para directors, and deactivators are known as meta directors.[9]Upon reacting, substituents will be added at the ortho, para or meta positions, depending on the directivity of the current substituents to make more complex benzene derivatives, often with several isomers. Electron flow leading to re-aromatization is key in ensuring the stability of such products.[9]
For example, threeisomersexist forcresolbecause the methyl group and the hydroxyl group (both ortho para directors) can be placed next to each other (ortho), one position removed from each other (meta), or two positions removed from each other (para).[10]Given that both the methyl and hydroxyl group are ortho-para directors, the ortho and para isomers are typically favoured.[10]Xylenolhas two methyl groups in addition to the hydroxyl group, and, for this structure, 6 isomers exist.[citation needed]
- Representative arene compounds
Arene rings can stabilize charges, as seen in, for example, phenol (C6H5–OH), which isacidicat the hydroxyl (OH), as charge on the oxygen (alkoxide –O−) is partially delocalized into the benzene ring.
Non-benzylic arenes
editAlthough benzylic arenes are common, non-benzylic compounds are also exceedingly important. Any compound containing a cyclic portion that conforms toHückel's ruleand is not a benzene derivative can be considered a non-benzylic aromatic compound.[5]
Monocyclic arenes
editOfannuleneslarger than benzene, [12]annulene and [14]annulene are weakly aromatic compounds and [18]annulene,Cyclooctadecanonaene,is aromatic, though strain within the structure causes a slight deviation from the precisely planar structure necessary for aromatic categorization.[11]Another example of a non-benzylic monocyclic arene is thecyclopropenyl(cyclopropenium cation), which satisfiesHückel's rulewith an n equal to 0.[12]Note, only the cationic form of this cyclic propenyl is aromatic, given that neutrality in this compound would violate either the octet rule orHückel's rule.[12]
Other non-benzylic monocyclic arenes include the aforementioned heteroarenes that can replace carbon atoms with other heteroatoms such as N, O or S.[5]Common examples of these are the five-memberedpyrroleand six-memberedpyridine,both of which have a substituted nitrogen[13]
Polycyclic aromatic hydrocarbons
editPolycyclic aromatic hydrocarbons,also known as polynuclear aromatic compounds (PAHs) are aromatic hydrocarbons that consist of fusedaromaticringsand do not containheteroatomsor carrysubstituents.[14]Naphthaleneis the simplest example of a PAH. PAHs occur inoil,coal,andtardeposits, and are produced as byproducts of fuel burning (whether fossil fuel or biomass).[15]As pollutants, they are of concern because some compounds have been identified ascarcinogenic,mutagenic,andteratogenic.[16][17][18][19]PAHs are also found in cooked foods.[15]Studies have shown that high levels of PAHs are found, for example, in meat cooked at high temperatures such as grilling or barbecuing, and in smoked fish.[15][16]They are also a goodcandidate molecule to act as a basis for the earliest forms of life.[20]Ingraphenethe PAH motif is extended to large 2D sheets.[21]
Reactions
editAromatic ring systems participate in many organic reactions.
Substitution
editIn aromaticsubstitution,onesubstituenton the arene ring, usually hydrogen, is replaced by another reagent.[5]The two main types areelectrophilic aromatic substitution,when the active reagent is an electrophile, andnucleophilic aromatic substitution,when the reagent is a nucleophile. Inradical-nucleophilic aromatic substitution,the active reagent is aradical.[22][23]
An example ofelectrophilic aromatic substitutionis the nitration ofsalicylic acid,where a nitro group is added para to the hydroxide substituent:
Nucleophilic aromatic substitutioninvolves displacement of aleaving group,such as ahalide,on anaromatic ring.Aromatic rings usually nucleophilic, but in the presence ofelectron-withdrawing groupsaromatic compounds undergo nucleophilic substitution. Mechanistically, this reaction differs from a commonSN2 reaction,because it occurs at a trigonal carbon atom (sp2hybridization).[24]
Hydrogenation
editHydrogenationof arenes create saturated rings. The compound1-naphtholis completely reduced to a mixture ofdecalin-olisomers.[25]
The compoundresorcinol,hydrogenated withRaney nickelin presence of aqueoussodium hydroxideforms anenolatewhich is alkylated withmethyl iodideto 2-methyl-1,3-cyclohexandione:[26]
Dearomatization
editIndearomatization reactionsthe aromaticity of the reactant is lost. In this regard, the dearomatization is related to hydrogenation. A classic approach isBirch reduction.The methodology is used in synthesis.[27]
See also
edit- Aromatic substituents:Aryl,AryloxyandArenediyl
- Asphaltene
- Hydrodealkylation
- Simple aromatic rings
- Rhodium-platinum oxide,a catalyst used to hydrogenate aromatic compounds.
References
edit- ^"Aromatic".IUPAC GoldBook.Retrieved2023-11-06.
- ^abSmith, Michael B.;March, Jerry(2007),Advanced Organic Chemistry: Reactions, Mechanisms, and Structure(6th ed.), New York: Wiley-Interscience,ISBN978-0-471-72091-1
- ^IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book" ). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. ISBN 0-9678550-9-8. https://doi.org/10.1351/goldbook.
- ^Balaban, Alexandru T.; Oniciu, Daniela C.; Katritzky, Alan R. (2004-05-01)."Aromaticity as a Cornerstone of Heterocyclic Chemistry".Chemical Reviews.104(5):2777–2812.doi:10.1021/cr0306790.ISSN0009-2665.PMID15137807.
- ^abcdefghijklKlein, David R. (2017).Organic Chemistry(3rd ed.). John Wiley & Sons.ISBN9781119444251.
- ^ab"Benzene | Definition, Discovery, Structure, Properties, & Uses | Britannica".britannica.Retrieved2023-11-06.
- ^Armit, James Wilson; Robinson, Robert (1925)."CCXI.—Polynuclear heterocyclic aromatic types. Part II. Some anhydronium bases".J. Chem. Soc., Trans.127:1604–1618.doi:10.1039/CT9252701604.ISSN0368-1645.
- ^abJensen, William B. (April 2009)."The Origin of the Circle Symbol for Aromaticity".Journal of Chemical Education.86(4): 423.Bibcode:2009JChEd..86..423J.doi:10.1021/ed086p423.ISSN0021-9584.
- ^abcd"16.5: An Explanation of Substituent Effects".Chemistry LibreTexts.2015-05-03.Retrieved2023-12-03.
- ^abBadanthadka, M.; Mehendale, H.M. (2014). "Cresols".Encyclopedia of Toxicology.pp.1061–1065.doi:10.1016/B978-0-12-386454-3.00296-7.ISBN978-0-12-386455-0.
- ^"What does" aromatic "really mean?".Chemistry LibreTexts.2013-10-02.Retrieved2023-11-06.
- ^ab"What does" aromatic "really mean?".Chemistry LibreTexts.2013-10-02.Retrieved2023-11-29.
- ^"4.2: Covalent Bonds".Chemistry LibreTexts.2020-07-30.Retrieved2023-11-06.
- ^Fetzer, John C. (2007-04-16)."THE CHEMISTRY AND ANALYSIS OF LARGE PAHs".Polycyclic Aromatic Compounds.27(2):143–162.doi:10.1080/10406630701268255.ISSN1040-6638.S2CID97930473.
- ^abc"Polycyclic Aromatic Hydrocarbons – Occurrence in foods, dietary exposure and health effects" (PDF). European Commission, Scientific Committee on Food. December 4, 2002. Archived (PDF) from the original on 2022-10-09.
- ^abLarsson, Bonny K.; Sahlberg, Greger P.; Eriksson, Anders T.; Busk, Leif A. (July 1983)."Polycyclic aromatic hydrocarbons in grilled food".Journal of Agricultural and Food Chemistry.31(4):867–873.Bibcode:1983JAFC...31..867L.doi:10.1021/jf00118a049.ISSN0021-8561.PMID6352775.
- ^Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on Marine Biotoxins in Shellfish – Saxitoxin Group. The EFSA Journal (2009) 1019, 1-76.
- ^Keith, Lawrence H. (2015-03-15)."The Source of U.S. EPA's Sixteen PAH Priority Pollutants".Polycyclic Aromatic Compounds.35(2–4):147–160.doi:10.1080/10406638.2014.892886.ISSN1040-6638.
- ^Thomas, Philippe J.; Newell, Emily E.; Eccles, Kristin; Holloway, Alison C.; Idowu, Ifeoluwa; Xia, Zhe; Hassan, Elizabeth; Tomy, Gregg; Quenneville, Cheryl (2021-02-01)."Co-exposures to trace elements and polycyclic aromatic compounds (PACs) impacts North American river otter (Lontra canadensis) baculum".Chemosphere.265:128920.Bibcode:2021Chmsp.26528920T.doi:10.1016/j.chemosphere.2020.128920.ISSN0045-6535.PMID33213878.
- ^Ehrenfreund, Pascale; Rasmussen, Steen; Cleaves, James; Chen, Liaohai (June 2006)."Experimentally Tracing the Key Steps in the Origin of Life: The Aromatic World".Astrobiology.6(3):490–520.Bibcode:2006AsBio...6..490E.doi:10.1089/ast.2006.6.490.ISSN1531-1074.PMID16805704.
- ^Wang, Xiao-Ye; Yao, Xuelin; Müllen, Klaus (2019-09-01)."Polycyclic aromatic hydrocarbons in the graphene era".Science China Chemistry.62(9):1099–1144.doi:10.1007/s11426-019-9491-2.hdl:21.11116/0000-0004-B547-0.ISSN1869-1870.S2CID198333072.
- ^"22.4: Electrophilic Aromatic Substitution".Chemistry LibreTexts.2014-11-26.Retrieved2023-11-29.
- ^"16.7: Nucleophilic Aromatic Substitution".Chemistry LibreTexts.2015-05-03.Retrieved2023-11-29.
- ^Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012-03-15).Organic Chemistry(Second ed.). Oxford, New York: Oxford University Press. pp.514–515.ISBN978-0-19-927029-3.
- ^Meyers, A. I.; Beverung, W. N.; Gault, R."1-Naphthol".Organic Syntheses.51:103;Collected Volumes,vol. 6.
- ^Noland, Wayland E.; Baude, Frederic J."Ethyl Indole-2-carboxylate".Organic Syntheses.41:56;Collected Volumes,vol. 5.
- ^Roche, Stéphane P.; Porco, John A. (2011-04-26)."Dearomatization Strategies in the Synthesis of Complex Natural Products".Angewandte Chemie International Edition.50(18):4068–4093.doi:10.1002/anie.201006017.ISSN1433-7851.PMC4136767.PMID21506209.
- ^Zheng, Chao; You, Shu-Li (2021-03-24)."Advances in Catalytic Asymmetric Dearomatization".ACS Central Science.7(3):432–444.doi:10.1021/acscentsci.0c01651.ISSN2374-7943.PMC8006174.PMID33791426.
External links
edit- Media related toaromatic compoundsat Wikimedia Commons