Contrast-enhanced ultrasound(CEUS) is the application ofultrasoundcontrast mediumto traditionalmedical sonography.Ultrasoundcontrast agentsrely on the different ways in which sound waves are reflected from interfaces between substances. This may be the surface of a small air bubble or a more complex structure. Commercially available contrast media are gas-filledmicrobubblesthat are administered intravenously to thesystemic circulation.Microbubbles have a high degree ofechogenicity(the ability of an object to reflect ultrasound waves). There is a great difference in echogenicity between the gas in the microbubbles and the softtissuesurroundings of the body. Thus,ultrasonic imagingusing microbubble contrast agents enhances the ultrasoundbackscatter,(reflection) of the ultrasound waves, to produce asonogramwith increased contrast due to the high echogenicity difference. Contrast-enhanced ultrasound can be used to image bloodperfusionin organs, measureblood flowrate in theheartand other organs, and for other applications.

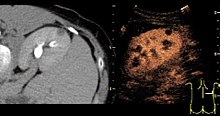
Targetingligandsthat bind toreceptorscharacteristic ofintravasculardiseases can be conjugated tomicrobubbles,enabling the microbubble complex to accumulate selectively in areas of interest, such asdiseasedor abnormal tissues. This form of molecular imaging, known as targeted contrast-enhanced ultrasound, will only generate a strong ultrasound signal if targeted microbubbles bind in the area of interest. Targeted contrast-enhanced ultrasound may have many applications in bothmedical diagnosticsand medical therapeutics. However, the targeted technique has not yet been approved by the FDA for clinical use in the United States.
Contrast-enhanced ultrasound is regarded as safe in adults, comparable to the safety ofMRI contrast agents,and better thanradiocontrast agentsused incontrast CT scans.The more limited safety data in children suggests that such use is as safe as in the adult population.[2]
Bubble echocardiogram
editAnechocardiogramis a study of theheartusing ultrasound. A bubble echocardiogram is an extension of this that uses simple air bubbles as a contrast medium during this study and often has to be requested specifically.
Although colour Doppler can be used to detect abnormal flows between the chambers of the heart (e.g.,persistent (patent) foramen ovale), it has a limitedsensitivity.When specifically looking for a defect such as this, small air bubbles can be used as a contrast medium and injected intravenously, where they travel to the right side of the heart. The test would be positive for an abnormal communication if the bubbles are seen passing into the left side of the heart. (Normally, they would exit theheartthrough thepulmonary arteryand be stopped by the lungs.) This form of bubble contrast medium is generated on anad hocbasis by the testing clinician by agitatingnormal saline(e.g., by rapidly and repeatedly transferring the saline between two connected syringes) immediately prior to injection.[citation needed]
Microbubble contrast agents
editGeneral features
editThere are a variety of microbubble contrast agents. Microbubbles differ in their shell makeup, gas core makeup, and whether or not they are targeted.[citation needed]
- Microbubble shell:selection of shell material determines how easily the microbubble is taken up by theimmune system.A morehydrophilicmaterial tends to be taken up more easily, which reduces the microbubble residence time in the circulation. This reduces the time available for contrast imaging. The shell material also affects microbubble mechanical elasticity. The more elastic the material, the more acoustic energy it can withstand before bursting.[3]Most commonly, microbubble shells are composed ofalbumin,galactose,lipid,orpolymers.[4]Hydrophobicparticles have also been applied to stabilize microbubble shells.[5]
- Microbubble gas core:The gas core is primary part of the ultrasound contrast microbubble that determines its echogenicity. Gas bubbles that are subjected to ultrasound pulsate and scatter a characteristic signal. This signal manifests itself as a high-amplitude entity in a contrast-enhanced sonogram. Gas cores can be composed ofair,or heavy gases likeperfluorocarbon,ornitrogen.[4]Heavy gases are less water-soluble so they are less likely to leak out from the microbubble leading to microbubble dissolution.[3]As a result, microbubbles with heavy gas cores last longer in circulation. To increaseharmonicpulsationbehavior, liquid and solid cores have been added to the gas contents.[6]
Regardless of the shell or gas core composition, microbubble size is fairly uniform. They lie within a range of 1–4 micrometres in diameter. That makes them smaller thanred blood cells,which allows them to flow easily through the circulation as well as the microcirculation.
Specific agents
edit- Perflutrenlipid microspheres (brand names Definity, Luminity) areperfluorocarbon emulsionscomposed ofoctafluoropropaneencapsulated in an outerlipid shell.[7][8]
- Octafluoropropanegas core with an albumin shell (Optison) is anotherperfluorocarbon emulsionthat is anFood and Drug Administration(FDA)-approved microbubble and made byGE Healthcare).
- Sulphur hexafluoridemicrobubbles(SonoVueBracco (company)). It is mainly used to characterize liver lesions that cannot be properly identified using conventional (b-mode) ultrasound. It remains visible in the blood for 3 to 8 minutes, and is expired by the lungs.[9]
- Airwithin a lipid/galactose shell (formerly Levovist, an FDA-approved microbubble that was made bySchering).[4]
- Perflexanelipid microspheres (formerly Imagent or Imavist) was an injectable suspension developed byAlliance Pharmaceuticalapproved by the FDA (in June 2002) for improving visualization of the left ventricular chamber of the heart, the delineation of the endocardial borders in patients with suboptimal echocardiograms. Beside its use to assess cardiac function and perfusion it is also used as an enhancer of the images of prostate, liver, kidney and other organs.[10]
Targeted microbubbles
editTargeted microbubbles are under preclinical development. They retain the same general features as untargeted microbubbles, but they are outfitted with ligands that bind specific receptors expressed by cell types of interest, such as inflamed cells or cancer cells. Current microbubbles in development are composed of a lipid monolayer shell with a perfluorocarbon gas core. The lipid shell is also covered with apolyethylene glycol(PEG) layer. PEG prevents microbubble aggregation and makes the microbubble more non-reactive. It temporarily "hides" the microbubble from the immune system uptake, increasing the amount of circulation time, and hence, imaging time.[11]In addition to the PEG layer, the shell is modified with molecules that allow for the attachment ofligandsthat bind certainreceptors.These ligands are attached to the microbubbles usingcarbodiimide,maleimide,or biotin-streptavidin coupling.[11]Biotin-streptavidin is the most popular coupling strategy becausebiotin'saffinity forstreptavidinis very strong and it is easy to label the ligands with biotin. Currently, these ligands aremonoclonal antibodiesproduced from animal cell cultures that bind specifically to receptors and molecules expressed by the target cell type. Since the antibodies are not humanized, they will elicit an immune response when used in human therapy. Humanizing antibodies is an expensive and time-intensive process, so it would be ideal to find an alternative source of ligands, such as synthetically manufactured targetingpeptidesthat perform the same function, but without the immune issues.[citation needed]
Types
editThere are two forms of contrast-enhanced ultrasound, untargeted (used in the clinic today) and targeted (under preclinical development). The two methods slightly differ from each other.
Untargeted CEUS
editUntargeted microbubbles, such as the aforementioned SonoVue, Optison, or Levovist, are injected intravenously into the systemic circulation in a small bolus. The microbubbles will remain in the systemic circulation for a certain period of time. During that time, ultrasound waves are directed on the area of interest. When microbubbles in the blood flow past the imaging window, the microbubbles'compressiblegas coresoscillatein response to the high frequency sonic energy field, as described in theultrasoundarticle. The microbubbles reflect a uniqueechothat stands in stark contrast to the surrounding tissue due to the orders of magnitude mismatch between microbubble and tissue echogenicity. The ultrasound system converts the strong echogenicity into a contrast-enhanced image of the area of interest. In this way, the bloodstream's echo is enhanced, thus allowing the clinician to distinguishbloodfrom surrounding tissues.[citation needed]
Targeted CEUS
editTargeted contrast-enhanced ultrasound works in a similar fashion, with a few alterations. Microbubbles targeted with ligands that bind certain molecular markers that are expressed by the area of imaging interest are still injected systemically in a small bolus. Microbubbles theoretically travel through the circulatory system, eventually finding their respective targets and binding specifically. Ultrasound waves can then be directed on the area of interest. If a sufficient number of microbubbles have bound in the area, their compressible gas cores oscillate in response to the high frequency sonic energy field, as described in theultrasoundarticle. The targeted microbubbles also reflect a unique echo that stands in stark contrast to the surrounding tissue due to the orders of magnitude mismatch between microbubble and tissue echogenicity. The ultrasound system converts the strong echogenicity into a contrast-enhanced image of the area of interest, revealing the location of the bound microbubbles.[12]Detection of bound microbubbles may then show that the area of interest is expressing that particular molecular marker, which can be indicative of a certain disease state, or identify particular cells in the area of interest.[citation needed]
Applications
editUntargeted contrast-enhanced ultrasound is currently applied inechocardiographyandradiology.Targeted contrast-enhanced ultrasound is being developed for a variety of medical applications.
Untargeted CEUS
editUntargeted microbubbles like Optison and Levovist are currently used in echocardiography. In addition, SonoVue[13]ultrasound contrast agent is used in radiology for lesion characterization.
- Organ Edge Delineation:microbubbles can enhance the contrast at the interface between the tissue and blood. A clearer picture of this interface gives the clinician a better picture of the structure of an organ. Tissue structure is crucial in echocardiograms, where a thinning, thickening, or irregularity in the heart wall indicates a serious heart condition that requires either monitoring or treatment.
- Blood Volume and Perfusion:contrast-enhanced ultrasound holds the promise for (1) evaluating the degree of blood perfusion in an organ or area of interest and (2) evaluating the blood volume in an organ or area of interest. When used in conjunction withDopplerultrasound, microbubbles can measure myocardial flow rate to diagnose valve problems. And the relative intensity of the microbubble echoes[14]can also provide a quantitative estimate on blood volume.
- Lesion Characterization:contrast-enhanced ultrasound plays a role in the differentiation between benign and malignant focal liver lesions. This differentiation relies on the observation[15]or processing[16][17]of the dynamic vascular pattern in a lesion with respect to its surrounding tissueparenchyma.
Targeted CEUS
edit- Inflammation:Contrast agents may be designed to bind to certain proteins that become expressed in inflammatory diseases such asCrohn's disease,atherosclerosis,and evenheart attacks.Cells of interest in such cases areendothelial cellsof blood vessels, andleukocytes:
- The inflamed blood vessels specifically express certain receptors, functioning ascell adhesion molecules,likeVCAM-1, ICAM-1, E-selectin.If microbubbles are targeted with ligands that bind these molecules, they can be used in contrast echocardiography to detect the onset of inflammation. Early detection allows the design of better treatments. Attempts have been made to outfit microbubbles with monoclonal antibodies that bindP-selectin,ICAM-1,andVCAM-1,[4]but the adhesion to the molecular target was poor and a large fraction of microbubbles that bound to the target rapidly detached, especially at high shear stresses ofphysiological relevance.[18]
- Leukocytespossess high adhesion efficiencies, partly due to a dual-ligandselectin-integrincell arrest system.[19]One ligand:receptor pair (PSGL-1:selectin) has a fast bond on-rate to slow the leukocyte and allows the second pair (integrin:immunoglobulinsuperfamily), which has a slower on-rate but slow off-rate to arrest the leukocyte, kinetically enhancing adhesion. Attempts have been made to make contrast agents bind to such ligands, with techniques such as dual-ligand targeting of distinct receptors to polymer microspheres,[20][21]andbiomimicryof the leukocyte's selectin-integrin cell arrest system,[22]having shown an increased adhesion efficiency, but yet not efficient enough to allow clinical use of targeted contrast-enhanced ultrasound for inflammation.
- Thrombosisandthrombolysis:Activatedplateletsare major components of blood clots (thrombi).Microbubblescan be conjugated to a recombinantsingle-chain variable fragmentspecific for activatedglycoprotein IIb/IIIa(GPIIb/IIIa), which is the most abundant platelet surface receptor. Despite the high shear stress at the thrombus area, the GPIIb/IIIa-targeted microbubbles will specifically bind to activated platelets and allow real-time molecular imaging of thrombosis, such as inmyocardial infarction,as well as monitoring success or failure of pharmacological thrombolysis.[23]
- Cancer:cancer cells also express a specific set of receptors, mainly receptors that encourageangiogenesis,or the growth of new blood vessels. If microbubbles are targeted with ligands that bind receptors likeVEGFor activatedglycoprotein IIb/IIIa,they can non-invasively and specifically identify areas of cancers.[24]
- Gene Delivery:Vector DNAcan be conjugated to the microbubbles. Microbubbles can be targeted with ligands that bind to receptors expressed by the cell type of interest. When the targeted microbubble accumulates at the cell surface with its DNA payload, ultrasound can be used to burst the microbubble. The force associated with the bursting may temporarily permeablize surrounding tissues and allow the DNA to more easily enter the cells. Targeted theranostic microbubbles (directed atVCAM-1) have been employed to deliver miR126 in a preclinical setting to stop the development ofAAAin vivo.[25]
- Drug Delivery:drugs can be incorporated into the microbubble's lipid shell. The microbubble's large size relative to other drug delivery vehicles likeliposomesmay allow a greater amount of drug to be delivered per vehicle. By targeted the drug-loaded microbubble with ligands that bind to a specific cell type, microbubble will not only deliver the drug specifically, but can also provide verification that the drug is delivered if the area is imaged using ultrasound.[26]Ultrasound-guided drug delivery has been successfully applied in the treatment ofpancreatic cancer.[27][28]
Advantages
editOn top of the strengths mentioned in themedical sonographyentry, contrast-enhanced ultrasound adds these additional advantages:
- The body is 73% water, and therefore, acoustically homogeneous. Blood and surrounding tissues have similar echogenicities, so it is also difficult to clearly discern the degree of blood flow, perfusion, or the interface between the tissue and blood using traditional ultrasound.[4]
- Ultrasound imaging allows real-time evaluation of blood flow.[29]
- Destruction of microbubbles by ultrasound[30]in the image plane allows absolute quantification of tissue perfusion.[31]
- Ultrasonic molecular imaging is safer than molecular imaging modalities such asradionuclide imagingbecause it does not involve radiation.[29]
- Alternative molecular imaging modalities, such asMRI,PET,andSPECTare very costly. Ultrasound, on the other hand, is very cost-efficient and widely available.[12]
- Since microbubbles can generate such strong signals, a lower intravenous dosage is needed, micrograms of microbubbles are needed compared to milligrams for other molecular imaging modalities such asMRI contrast agents.[12]
- Targeting strategies for microbubbles are versatile and modular. Targeting a new area only entails conjugating a new ligand.
- Active targeting can be increased (enhanced microbubbles adhesion) byAcoustic radiation force[32][33]using a clinical ultrasound imaging system in 2D-mode[34][35]and 3D-mode.[36]
Disadvantages
editIn addition to the weaknesses mentioned in themedical sonographyentry, contrast-enhanced ultrasound has the following disadvantages:
- Microbubbles don't last very long in circulation. They have low circulation residence times because they either get taken up by immune system cells or get taken up by theliverorspleeneven when they are coated with PEG.[12]
- Ultrasound produces more heat as the frequency increases, so the ultrasonic frequency must be carefully monitored.
- Microbubbles burst at low ultrasound frequencies and at high mechanical indices (MI), which is the measure of the negative acoustic pressure of the ultrasound imaging system. Increasing MI increases image quality, but there are tradeoffs with microbubble destruction. Microbubble destruction could cause local microvasculature ruptures andhemolysis.[11]
- Targeting ligands can be immunogenic, since current targeting ligands used in preclinical experiments are derived fromanimal culture.[11]
- Low targeted microbubble adhesion efficiency, which means a small fraction of injected microbubbles bind to the area of interest.[18]This is one of the main reasons that targeted contrast-enhanced ultrasound remains in the preclinical development stages.
See also
edit
References
edit- ^abContent initially copied from:Hansen, Kristoffer; Nielsen, Michael; Ewertsen, Caroline (2015)."Ultrasonography of the Kidney: A Pictorial Review".Diagnostics.6(1): 2.doi:10.3390/diagnostics6010002.ISSN2075-4418.PMC4808817.PMID26838799.(CC-BY 4.0)
- ^Sidhu, Paul; Cantisani, Vito; Deganello, Annamaria; Dietrich, Christoph; Duran, Carmina; Franke, Doris; Harkanyi, Zoltan; Kosiak, Wojciech; Miele, Vittorio; Ntoulia, Aikaterini; Piskunowicz, Maciej; Sellars, Maria; Gilja, Odd (2016)."Role of Contrast-Enhanced Ultrasound (CEUS) in Paediatric Practice: An EFSUMB Position Statement".Ultraschall in der Medizin – European Journal of Ultrasound.38(1): 33–43.doi:10.1055/s-0042-110394.ISSN0172-4614.PMID27414980.
- ^abMcCulloch M.; Gresser C.; Moos S.; Odabashian J.; Jasper S.; Bednarz J.; Burgess P.; Carney D.; Moore V.; Sisk E.; Waggoner A.; Witt S.; Adams D. (2000). "Ultrasound contrast physics: A series on contrast echocardiography, article 3".J Am Soc Echocardiogr.13(10): 959–67.doi:10.1067/mje.2000.107004.PMID11029724.
- ^abcdeLindner J.R. (2004). "Microbubbles in medical imaging: current applications and future directions".Nat Rev Drug Discov.3(6): 527–32.doi:10.1038/nrd1417.PMID15173842.S2CID29807146.
- ^Anderton N, Carlson CS, Matsumoto R, Shimizu RI, Poortinga AT, Kudo N, Postema M (2022)."On the rigidity of four hundred Pickering-stabilised microbubbles".Japanese Journal of Applied Physics.61(SG): SG8001.Bibcode:2022JaJAP..61G8001A.doi:10.35848/1347-4065/ac4adc.S2CID245915590.
- ^Panfilova A, Chen P, van Sloun R, Wijkstra H, Postema M, Mischi M (2021)."Experimental acoustic characterisation of an endoskeletal antibubble contrast agent: first results".Medical Physics.48(11): 6765–6780.Bibcode:2021MedPh..48.6765P.doi:10.1002/mp.15242.PMC9293338.PMID34580883.
- ^"Definity- perflutren injection, suspension".DailyMed.19 August 2020.Retrieved22 October2020.
- ^"Luminity EPAR".European Medicines Agency(EMA).17 September 2018.Retrieved22 October2020.
- ^"SonoVue, INN-sulphur hexafluoride - Annex I - Summary of Product Characteristics"(PDF).European Medicines Agency.Retrieved2019-02-24.
- ^"Perflexane: (AF0150, AFO 150, Imagent, Imavist™)".Drugs in R&D,Volume 3, Number 5, 2002, pp. 306–309(4).Adis International.Retrieved2010-03-08.
- ^abcdKlibanov A.L. (2005). "Ligand-carrying gas-filled microbubbles: ultrasound contrast agents for targeted molecular imaging".Bioconjug. Chem.16(1): 9–17.doi:10.1021/bc049898y.PMID15656569.
- ^abcdKlibanov A.L. (1999). "Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging".Adv Drug Deliv Rev.37(1–3): 139–157.doi:10.1016/s0169-409x(98)00104-5.PMID10837732.
- ^Schneider, M (November 1999). "SonoVue, a new ultrasound contrast agent".Eur. Radiol.9(3 Supplement): S347–S348.doi:10.1007/pl00014071.PMID10602926.S2CID19613214.
- ^Rognin, NG; Frinking, P.; Costa, M.; Arditi, M. (November 2008). "In-vivo perfusion quantification by contrast ultrasound: Validation of the use of linearized video data vs. Raw RF data".2008 IEEE Ultrasonics Symposium.pp. 1690–1693.doi:10.1109/ULTSYM.2008.0413.ISBN978-1-4244-2428-3.S2CID45679140.
- ^Claudon, M; Dietrich, CF.; Choi, BI.; Cosgrove, DO.; Kudo, M.; Nolsøe, CP.; Piscaglia, F.; Wilson, SR.; Barr, RG.; Chammas, MC.; Chaubal, NG.; Chen, MH.; Clevert, DA.; Correas, JM.; Ding, H.; Forsberg, F.; Fowlkes, JB.; Gibson, RN.; Goldberg, BB.; Lassau, N.; Leen, EL.; Mattrey, RF.; Moriyasu, F.; Solbíatí, L.; Weskott, HP.; Xu, HX (February 2013)."Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver-Update 2012: A WFUMB-EFSUMB Initiative in Cooperation With Representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS".Ultrasound Med Biol.39(2): 187–210.doi:10.1016/j.ultrasmedbio.2012.09.002.hdl:11585/144895.PMID23137926.S2CID2224370.
- ^Rognin, NG; Arditi, M.; Mercier, L.; Frinking, P.J.A.; Schneider, M.; Perrenoud, G.; Anaye, A.; Meuwly, J.; Tranquart, F. (November 2010). "Parametric imaging for characterizing focal liver lesions in contrast-enhanced ultrasound".IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control.57(11): 2503–2511.doi:10.1109/TUFFC.2010.1716.PMID21041137.S2CID19339331.
- ^Anaye, A; Perrenoud, G.; Rognin, N.; Arditi, M.; Mercier, L.; Frinking, P.; Ruffieux, C.; Peetrons, P.; Meuli, R.; Meuwly, JY. (October 2011). "Differentiation of focal liver lesions: usefulness of parametric imaging with contrast-enhanced US".Radiology.261(1): 300–310.doi:10.1148/radiol.11101866.PMID21746815.
- ^abTakalkar A.M.; Klibanov A.L.; Rychak J.J.; Lindner J.R.; Ley K. (2004). "Binding and detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow".J. Control. Release.96(3): 473–482.doi:10.1016/j.jconrel.2004.03.002.PMID15120903.
- ^Eniola A.O.; Willcox P.J.; Hammer D.A. (2003)."Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs".Biophys. J.85(4): 2720–31.Bibcode:2003BpJ....85.2720E.doi:10.1016/s0006-3495(03)74695-5.PMC1303496.PMID14507735.
- ^Eniola A.O.; Hammer D.A. (2005). "In vitro characterization of leukocyte mimetic for targeting therapeutics to the endothelium using two receptors".Biomaterials.26(34): 7136–44.doi:10.1016/j.biomaterials.2005.05.005.PMID15953632.
- ^Weller G.E.; Villanueva F.S.; Tom E.M.; Wagner W.R. (2005). "Targeted ultrasound contrast agents: In vitro assessment of endothelial dysfunction and multi-targeting to ICAM-1 and sialyl Lewis(x)".Biotechnol. Bioeng.92(6): 780–8.doi:10.1002/bit.20625.PMID16121392.
- ^Rychak J.J., A.L. Klibanov, W. Yang, B. Li, S. Acton, A. Leppanen, R.D. Cummings, and K. Ley. "Enhanced Microbubble Adhesion to P-selectin with a Physiologically-tuned Targeting Ligand," 10th Ultrasound Contrast Research Symposium in Radiology, San Diego, CA, March 2005.
- ^Wang, X; Hagemeyer, CE; Hohmann, JD; Leitner, E; Armstrong, PC; Jia, F; Olschewski, M; Needles, A; Peter, K; Ingo, A (June 2012)."Novel single-chain antibody-targeted microbubbles for molecular ultrasound imaging of thrombosis: Validation of a unique non-invasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice".Circulation.125(25): 3117–3126.doi:10.1161/CIRCULATIONAHA.111.030312.PMID22647975.
- ^Yap, ML; McFadyen, JD; Wang, X; Hohmann, JD; Ziegler, M; Yao, Y; Pham, A; Harris, M; Donnelly, PS; Hogarth, PM; Pietersz, GA; Lim, B; Peter, K (June 2017)."Targeting Activated Platelets: A Unique and Potentially Universal Approach for Cancer Imaging".Theranostics.7(10): 2565–2574.doi:10.7150/thno.19900.PMC5558553.PMID28819447.
- ^Wang, X; Searle, AK; Hohmann, JD; Liu, AO; Abraham, MK; Palasubramaniam, J; Lim, B; Yao, Y; Wallert, M; Yu, E; Chen, YC; Peter, K (April 2018)."Dual-Targeted Theranostic Delivery of miRs Arrests Abdominal Aortic Aneurysm Development".Molecular Therapy.25(4): 1056–1065.doi:10.1016/j.ymthe.2018.02.010.PMC6080135.PMID2952574.
- ^Wang, X; Gkanatsas, Y; Palasubramaniam, J; Hohmann, JD; Chen, YC; Lim, B; Hagemeyer, CE; Peter, K (March 2016)."Thrombus-Targeted Theranostic Microbubbles: A New Technology towards Concurrent Rapid Ultrasound Diagnosis and Bleeding-free Fibrinolytic Treatment of Thrombosis".Theranostics.6(5): 726–738.doi:10.7150/thno.14514.PMC4805666.PMID27022419.
- ^Dimcevski G, Kotopoulis S, Bjånes T, Hoem D, Schjött J, Gjertsen BT, Biermann M, Molven A, Sorbye H, Mc Cormack E, Postema M, Gilja OH (2016)."A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer".Journal of Controlled Release.243:172–181.doi:10.1016/j.jconrel.2016.10.007.hdl:1956/16668.PMID27744037.
- ^Søreide K, Stättner S (2021). Søreide K, Stättner S (eds.).Textbook of Pancreatic Cancer: Principles and Practice of Surgical Oncology.Heidelberg: Springer.doi:10.1007/978-3-030-53786-9.ISBN978-3-030-53786-9.S2CID231793989.
- ^abLindner, J.R., A.L. Klibanov, and K. Ley. Targeting inflammation, In: Biomedical aspects of drug targeting. (Muzykantov, V.R.,Torchilin, V.P.,eds.) Kluwer, Boston, 2002; pp. 149–172.
- ^Wei, K; Jayaweera, AR; Firoozan, S; Linka, A; Skyba, DM; Kaul, S (February 1998)."Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion".Circulation.97(5): 473–483.doi:10.1161/01.cir.97.5.473.PMID9490243.
- ^Arditi, M; Frinking, PJA.; Zhou, X.; Rognin, NG. (June 2006). "A new formalism for the quantification of tissue perfusion by the destruction-replenishment method in contrast ultrasound imaging".IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control.53(6): 1118–1129.doi:10.1109/TUFFC.2006.1642510.PMID16846144.S2CID6328131.
- ^Rychak, JJ; Klibanov, AL; Hossack, JA (March 2005). "Acoustic radiation force enhances targeted delivery of ultrasound contrast microbubbles: in vitro verification".IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control.52(3): 421–433.doi:10.1109/TUFFC.2005.1417264.PMID15857050.S2CID25032596.
- ^Dayton, P; Klibanov, A; Brandenburger, G; Ferrara, K (October 1999)."Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles".Ultrasound Med Biol.25(8): 1195–1201.doi:10.1016/s0301-5629(99)00062-9.PMID10576262.
- ^Frinking, PJ; Tardy, I; Théraulaz, M; Arditi, M; Powers, J; Pochon, S; Tranquart, F (August 2012)."Effects of acoustic radiation force on the binding efficiency of BR55, a VEGFR2-specific ultrasound contrast agent".Ultrasound Med Biol.38(8): 1460–1469.doi:10.1016/j.ultrasmedbio.2012.03.018.PMID22579540.
- ^Gessner, RC; Streeter, JE; Kothadia, R; Feingold, S; Dayton, PA (April 2012)."An in vivo validation of the application of acoustic radiation force to enhance the diagnostic utility of molecular imaging using 3-d ultrasound".Ultrasound Med Biol.38(4): 651–660.doi:10.1016/j.ultrasmedbio.2011.12.005.PMC3355521.PMID22341052.
- ^Rognin, NG; Unnikrishnan, S.; Klibanov, AL. (September 2013)."Molecular Ultrasound Imaging Enhancement by Volumic Acoustic Radiation Force (VARF): Pre-clinical in vivo Validation in a Murine Tumor Model".Abstracts of the 2013 World Molecular Imaging Congress.Archived fromthe originalon 2013-10-11.