Inpopulation genetics,directional selectionis a type ofnatural selectionin which one extremephenotypeis favored over both the other extreme and moderate phenotypes. This genetic selection causes theallele frequencyto shift toward the chosen extreme over time as allele ratios change from generation to generation. The advantageous extreme allele will increase in frequency among the population as a consequence of survival and reproduction differences among the different present phenotypes in the population. The allele fluctuations as a result of directional selection can be independent of the dominance of the allele, and in some cases if the allele isrecessive,it can eventually becomefixedin the population.[1][2]
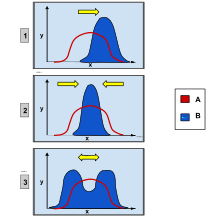
Directional selection was first identified and described by naturalistCharles Darwinin his bookOn the Origin of Speciespublished in 1859.[3]He identified it as a type of natural selection along withstabilizing selectionanddisruptive selection.[4]These types of selection also operate by favoring a specific allele and influencing the population's future phenotypic ratio. Disruptive selection favors both extreme phenotypes while the moderate phenotype will be selected against. The frequency of both extreme alleles will increase while the frequency of the moderate allele will decrease, differing from the trend in directional selection in which only one extreme allele is favored. Stabilizing selection favors the moderate phenotype and will select against both extreme phenotypes.[5]Directional selection can be observed infinchbeak size, peppered moth color, African cichlid mouth types, and sockeye salmon migration periods.
If there is continuous allele frequency change as a result of directional selection generation from generation, there will be observable changes in the phenotypes of the entire population over time. Directional selection can change the genotypic and phenotypic variation of a population and cause a trend toward one specific phenotype.[6]This selection is an important mechanism in the selection of complex and diversifying traits, and is also a primary force of speciation.[7]Changes in a genotype and consequently a phenotype can either be advantageous, harmful, or neutral and depend on the environment in which the phenotypic shift is happening.[8]
Evidence
editDetection methods
editDirectional selection most often occurs during environmental changes or population migrations to new areas with different environmental pressures. Directional selection allows for swift changes inallele frequencythat can accompany rapidly changing environmental factors and plays a major role in speciation.[7]Analysis on quantitative trait locus (QTL) effects has been used to examine the impact of directional selection in phenotypic diversification. QTL is a region of a gene that corresponds to a specific phenotypic trait, and the measuring the statistical frequencies of the traits can be helpful in analyzing phenotypic trends.[9]In one study, the analysis showed that directional changes in QTLs affecting various traits were more common than expected by chance among diverse species. This was an indication that directional selection is a primary cause of the phenotypic diversification that can eventually result in speciation.[10]
There are different statistical tests that can be run to test for the presence of directional selection in a population. A highly indicative test of changes in allele frequencies is the QTL sign test, and other tests include the Ka/Ks ratio test and the relative rate test. The QTL sign test compares the number of antagonistic QTL to a neutral model, and allows for testing of directional selection againstgenetic drift.[11]TheKa/Ks ratiotest compares the number of non-synonymous to synonymous substitutions, and a ratio that is greater than 1 indicates directional selection.[12]The relative ratio test looks at the accumulation of advantageous traits against a neutral model, but needs a phylogenetic tree for comparison. This can prove difficult if the full phylogenic history is not known or is not specific enough for the test comparison.[13]
Examples
editFinch beak size
editAnother example of directional selection is the beak size in a specific population offinches.Darwin first observed this in the publication of his book,On the Origin of Species,and he details how the size of the finches beak differs based on environmental factors. On theGalápagos Islandswest of the coast ofEcuador, there were groups of finches displaying different beak phenotypes.[14]In one group, the beaks ranged from large and tough to small and smooth. Throughout the wet years, small seeds were more common than large seeds, and because of the large supply of small seeds the finches rarely ate large seeds. During the dry years, neither the small or large seeds were in great abundance, and the birds trended towards eating larger seeds. The changes in diet of the finches based on the environmental wet and dry seasons affected the depth of the birds’ beaks in future generations.[15]The beaks most beneficial to the more plentiful type of seed would be selected for because the birds were able to feed themselves and reproduce.
Peppered moths
editA significant example of directional selection in populations is the fluctuations of light and dark phenotypes inpeppered mothsin the 1800s.[16]During the industrial revolution, environmental conditions were rapidly changing with the newfound emission of dark, black smoke from factories that would change the color of trees, rocks, and other niches of moths.[17]Before the industrial revolution, the most prominent phenotype in the peppered moth population was the lighter, speckled moths. They thrived on the light birch trees and their phenotype would provide them with better camouflage from predators. After theIndustrial Revolutionas the trees become darker with soot, the moths with the darker phenotype were able to blend in and avoid predators better than their white counterparts. As time went on, the darker moths were positively directionally selected for and the allele frequency began to shift due to the increase in the number of darker moths.[18]
African cichlids
editAfrican cichlidsare known to be a diverse fish species, with evidence indicating that they evolved extremely quickly. These fish evolved within the same habitat, but have a variety ofmorphologies,especially pertaining to the mouth and jaw. Experiments pertaining the cichlid jaw phenotypes was done by Albertson and others in 2003 by crossing two species of African cichlids with very different mouth morphologies. The cross betweenLabeotropheus fuelleborni(subterminal mouth for biting algae off rocks) andMetriaclima zebra(terminal mouth for suction feeding) allowed for mapping of QTLs affecting feeding morphology. Using the QTL sign test, definitive evidence was used to support the existence of directional selection in the oral jaw apparatus in African cichlids. However, this was not the case for the suspensorium or skull QTLs, suggesting genetic drift or stabilizing selection as mechanisms for the speciation.[19]
Sockeye salmon
editSockeye salmonare one of the many species of fish that areanadromous,in which individuals migrate to the same rivers in which they were born to reproduce. These migrations happen around the same time every year, but a 2007 study shows that sockeye salmon found in the waters of theBristol BayinAlaskahave recently undergone directional selection on the timing of migration.[20]In this study, two populations of sockeye salmon,EgegikandUgashik,were observed. Data from 1969–2003 provided by theAlaska Department of Fish and Gamewere divided into five sets of seven years and plotted for average arrival to the fishery. After analyzing the data, it was determined that in both populations the average migration date was earlier and the populations were undergoing directional selection as a result of changing ecological conditions. The Egegik population experienced stronger selection and the migration date shifted four days. The paper suggests that fisheries can be a factor driving this selection because fishing occurs more often in the later periods of migration (especially in the Egegik district), preventing those fish from reproducing.[21]This discovery also goes to show that in addition to environmental changes, human behaviors can also have massive effects on the selection of species around them.[22]
Bears hunting sockeye salmon
editStudies carried out in LittleTogiak Lakein Alaska, indicate that bearpredationhas a significant impact on sockeye salmon populations, especially in shallow streams. Bears often focus on larger male salmon and tend to prefer those that have just arrived at the spawning grounds, particularly in smaller streams where they can catch them more easily. This predation may accelerate the aging of salmon by favoring later arrivals. Additionally, the impact of predation varies among different salmon populations based on their habitat and density; it tends to be more selective in areas where fish are readily accessible. While high levels of bear predation can occur, healthy salmon populations usually maintain strong reproductive potential, although the effects are more pronounced when populations are low. Overall, these dynamics illustrate how bear predation affects salmon behavior and life cycles, influencing their evolutionary processes.[23]
Large Felids
editThis study examines the role of lineage-specific directional selection on body size evolution in felids, revealing that several species, including those in thePantheragenus (lions, tigers, leopards, jaguars, snow leopards), the cheetah, and the puma, exhibit evidence of directional selection favoring larger body mass. These larger body sizes are likely linked to hunting large prey and solitary hunting strategies, which favor physical strength and size. Conversely, the clouded leopard did not show evidence of directional selection for body size, suggesting different ecological pressures, and the jaguarundi showed no clear selection for smaller size despite being smaller than its relatives. These findings highlight that body size evolution in felids is not uniform and is strongly influenced by ecological factors such as prey size and hunting behavior. The study concludes that directional selection for increased body size is likely associated with the need for larger predators to capture large prey, and solitary hunting may accelerate this selection, although the evolutionary paths for different felid lineages can vary considerably.[24]
Soapberry Bugs
editSoapberry Bugs(Jadera haematoloma) primarily feed on seeds produced by plants of theSapindaceaefamily. These soapberry bugs use their beaks to feed on the seeds within the fruits of these plants, so it is crucial that their beak size is long enough to reach the seeds from the exterior of the fruits. However, the distance from the exterior of the fruit to the seed can vary. Scott Carroll and Christin Boyd (1992) conducted an experiment where they would observe how three newly introduced plant species introduced to North America that were colonized by these soapberry bugs would affect the natural selection of the insect’s beak length. Each new plant species hosted fruits of different sizes compared to the native hosts. They found that there was indeed a close correlation between the radius of the fruit and the length of the beak. There was a positive directional selection for larger beaks when the radius of the fruit was larger, and there was a positive directional selection for smaller beaks when the radius of the fruit was smaller. To confirm that these differences were caused by genetic differences and not through phenotypic plasticity, Carroll raised young soapberry bugs from the populations based on the introduced plant species and found that their beak length was retained when they were developed on the alternative host.[25]
Ecological impact
editDirectional selection can quickly lead to vast changes in allele frequencies in a population because of the cumulative nature of reproduction of the fittest. Because the main cause for directional selection is different and changing environmental pressures, rapidly changing environments, such as those affected byclimate change,can cause drastic changes within populations.
Diversity
editLimiting the number of genotypes in a certain population can be deleterious to the ecosystem as a whole by shrink the potential geneticgene pool.[26]Low amount of genetic variation can lead tomass extinctionsandendangered speciesbecause of the large impact one mutation can have on the entire population if there are only a few specific genes present throughout.
Urban Influence
editIt is important to note the impact that humans have on genetic diversity as well, and be aware of the ways to reduce harmful impacts on natural environments.[27]Major roads, waterway pollution, andurbanizationall cause environmental selection and could potentially result in changes in allele frequencies.[28]Hunting may also play a role in directional selection, albeit more so in smaller populations.
Timescale
editTypically directional selection acts strongly for short bursts and is not sustained over long periods of time.[29]If it was sustained, a population might hitbiological constraintssuch that it no longer responds to selection. However, it is possible for directional selection to take a very long time to find alocal optimumon afitness landscape.[30]A possible example of long-term directional selection is the tendency ofproteinsto become morehydrophobicover time,[31]and to have their hydrophobic amino acids more interspersed along thesequence.[32]
See also
editReferences
edit- ^Molles, MC (2010).Ecology Concepts and Applications.McGraw-Hill Higher Learning.
- ^Teshima, Kosuke M.; Przeworski, Molly (January 2006)."Directional Positive Selection on an Allele of Arbitrary Dominance".Genetics.172(1): 713–718.doi:10.1534/genetics.105.044065.PMC1456198.PMID16219788.
- ^Kaiser, Margaret (November 2014)."First editions of Darwin's 'Origin of Species'".National Library of Medicine.
- ^Darwin, C (1859).On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life.London: John Murray.
- ^Mitchell-Olds, Thomas; Willis, John H.; Goldstein, David B. (2007). "Which evolutionary processes influence natural genetic variation for phenotypic traits?".Nature Reviews Genetics.8(11). Springer Nature: 845–856.doi:10.1038/nrg2207.ISSN1471-0056.PMID17943192.S2CID14914998.
- ^Melo, Diogo; Marroig, Gabriel (January 2015)."Directional selection can drive the evolution of modularity in complex traits".Proceedings of the National Academy of Sciences of the United States of America.112(2): 470–475.Bibcode:2015PNAS..112..470M.doi:10.1073/pnas.1322632112.PMC4299217.PMID25548154.
- ^abRieseberg, Loren H.; Widmer, Alex; Arntz, A. Michele; Burke, John M. (September 2002)."Directional selection is the primary cause of phenotypic diversification".Proceedings of the National Academy of Sciences.99(19): 12242–12245.Bibcode:2002PNAS...9912242R.doi:10.1073/pnas.192360899.PMC129429.PMID12221290.
- ^Thiltgen, Grant; dos Reis, Mario; Goldstein, Richard A. (December 2016)."Finding Direction in the Search for Selection".Journal of Molecular Evolution.84(1): 39–50.doi:10.1007/s00239-016-9765-5.PMC5253163.PMID27913840.
- ^Powder, Kara E. (March 2024). "Quantitative Trait Loci (QTL) Mapping".EQTL Analysis.Methods in Molecular Biology. Vol. 2082. pp. 211–229.doi:10.1007/978-1-0716-0026-9_15.ISBN978-1-0716-0025-2.PMID31849018.
- ^Rieseberg, Loren H.; Widmer, Alex; Arntz, A. Michele; Burke, John M. (2002-09-17)."Directional selection is the primary cause of phenotypic diversification".Proceedings of the National Academy of Sciences of the United States of America.99(19): 12242–5.Bibcode:2002PNAS...9912242R.doi:10.1073/pnas.192360899.PMC129429.PMID12221290.
- ^Orr, H.A. (1998)."Testing Natural Selection vs. Genetic Drift in Phenotypic Evolution Using Quantitative Trait Locus Data".Genetics.149(4): 2099–2104.doi:10.1093/genetics/149.4.2099.PMC1460271.PMID9691061.
- ^Hurst, Laurence D (2002). "The Ka/Ks ratio: diagnosing the form of sequence evolution".Trends in Genetics.18(9). Elsevier BV: 486–487.doi:10.1016/s0168-9525(02)02722-1.ISSN0168-9525.PMID12175810.
- ^Creevey, Christopher J.; McInerney, James O. (2002). "An algorithm for detecting directional and non-directional positive selection, neutrality and negative selection in protein coding DNA sequences".Gene.300(1–2). Elsevier BV: 43–51.doi:10.1016/s0378-1119(02)01039-9.ISSN0378-1119.PMID12468084.
- ^Burrows, Leah (November 2021)."For Darwin's finches, beak shape goes beyond evolution".Harvard School of Engineering.
- ^Campbell, Neil A.; Reece, Jane B. (2002).Biology(6th ed.). Benjamin Cummings. pp. 450–451.ISBN978-0-8053-6624-2.
- ^"Peppered Moth".globalchange.umich.edu.Retrieved2024-03-24.
- ^"Peppered Moth and natural selection".butterfly-conservation.org.Retrieved2024-03-24.
- ^Saccheri, Ilik J. (October 2008)."Selection and gene flow on a diminishing cline of melanic peppered moths".Proceedings of the National Academy of Sciences.105(42): 16212–16217.Bibcode:2008PNAS..10516212S.doi:10.1073/pnas.0803785105.PMC2571026.PMID18854412.
- ^Albertson, R. C.; Streelman, J. T.; Kocher, T. D. (2003-04-18)."Directional selection has shaped the oral jaws of Lake Malawi cichlid fishes".Proceedings of the National Academy of Sciences.100(9): 5252–5257.Bibcode:2003PNAS..100.5252A.doi:10.1073/pnas.0930235100.ISSN0027-8424.PMC154331.PMID12704237.
- ^Quinn, Thomas P. (April 2007)."Directional selection by fisheries and the timing of sockeye salmon (Oncorhynchus Nerka) Migrations".Ecological Applications.17(3): 731–739.Bibcode:2007EcoAp..17..731Q.doi:10.1890/06-0771.PMID17494392.
- ^Quinn, Thomas P.; Hodgson, Sayre; Flynn, Lucy; Hilborn, Ray; Rogers, Donald E. (2007). "Directional Selection by Fisheries and the Timing of Sockeye Salmon (Oncorhynchus Nerka) Migrations".Ecological Applications.17(3). Wiley: 731–739.Bibcode:2007EcoAp..17..731Q.doi:10.1890/06-0771.ISSN1051-0761.PMID17494392.
- ^Lin, J. E.; Hard, J. J.; Naish, K. A.; Peterson, D.; Hilborn, R.; Hauser, L. (May 2016)."It's a bear market: evolutionary and ecological effects of predation on two wild sockeye salmon populations".Heredity.116(5): 447–457.doi:10.1038/hdy.2016.3.ISSN1365-2540.PMC4834386.
- ^Lin, J. E.; Hard, J. J.; Naish, K. A.; Peterson, D.; Hilborn, R.; Hauser, L. (May 2016)."It's a bear market: evolutionary and ecological effects of predation on two wild sockeye salmon populations".Heredity.116(5): 447–457.doi:10.1038/hdy.2016.3.ISSN1365-2540.PMC4834386.
- ^Harano, Tomohiro; Kutsukake, Nobuyuki (2023-03-01)."Way to big cats: Directional selection in body size evolution in living felids".Journal of Mammalian Evolution.30(1): 97–108.doi:10.1007/s10914-022-09639-z.ISSN1573-7055.
- ^Carroll, Scott P.; Boyd, Christin (1 August 1992)."HOST RACE RADIATION IN THE SOAPBERRY BUG: NATURAL HISTORY WITH THE HISTORY".Evolution.46(4): 1052–1069.doi:10.1111/j.1558-5646.1992.tb00619.x.ISSN0014-3820.
- ^Star, Bastiaan; Spencer, Hamish G. (May 2013)."Effects of Genetic Drift and Gene Flow on the Selective Maintenance of Genetic Variation".Genetics.194(1): 235–244.doi:10.1534/genetics.113.149781.PMC3632471.PMID23457235.
- ^Mysterud, Atle (13 May 2011)."Selective harvesting of large mammals: how often does it result in directional selection?".Journal of Applied Ecology.48(4): 827–834.doi:10.1111/j.1365-2664.2011.02006.x.
- ^Hunter, Philip (April 2007)."The human impact on biological diversity".EMBO Reports.8(4): 316–318.doi:10.1038/sj.embor.7400951.PMC1852758.PMID17401404.
- ^Hoekstra, H. E.; Hoekstra, J. M.; Berrigan, D.; Vignieri, S. N.; Hoang, A.; Hill, C. E.; Beerli, P.; Kingsolver, J. G. (2001-07-24)."Strength and tempo of directional selection in the wild".Proceedings of the National Academy of Sciences.98(16): 9157–9160.Bibcode:2001PNAS...98.9157H.doi:10.1073/pnas.161281098.ISSN0027-8424.PMC55389.PMID11470913.
- ^Kaznatcheev, Artem (May 2019)."Computational Complexity as an Ultimate Constraint on Evolution".Genetics.212(1): 245–265.doi:10.1534/genetics.119.302000.PMC6499524.PMID30833289.
- ^Wilson, Benjamin A.; Foy, Scott G.; Neme, Rafik; Masel, Joanna (24 April 2017)."Young genes are highly disordered as predicted by the preadaptation hypothesis of de novo gene birth"(PDF).Nature Ecology & Evolution.1(6): 0146–146.Bibcode:2017NatEE...1..146W.doi:10.1038/s41559-017-0146.hdl:10150/627822.PMC5476217.PMID28642936.
- ^Foy, Scott G.; Wilson, Benjamin A.; Bertram, Jason; Cordes, Matthew H. J.; Masel, Joanna (April 2019)."A Shift in Aggregation Avoidance Strategy Marks a Long-Term Direction to Protein Evolution".Genetics.211(4): 1345–1355.doi:10.1534/genetics.118.301719.PMC6456324.PMID30692195.
Further reading
edit- Sabeti PC; et al. (2006). "Positive Natural Selection in the Human Lineage".Science.312(5780): 1614–1620.Bibcode:2006Sci...312.1614S.doi:10.1126/science.1124309.PMID16778047.S2CID10809290.
- Pickrell JK, Coop G, Novembre J, et al. (May 2009)."Signals of recent positive selection in a worldwide sample of human populations".Genome Research.19(5): 826–837.doi:10.1101/gr.087577.108.PMC2675971.PMID19307593.
- Types of Selection
- Natural Selection
- Modern Theories of Evolution