Famotidine,sold under the brand namePepcidamong others, is ahistamine H2receptor antagonistmedication that decreasesstomach acidproduction.[4]It is used to treatpeptic ulcer disease,gastroesophageal reflux disease,andZollinger-Ellison syndrome.[4]It is takenby mouthor byinjection into a vein.[4]It begins working within an hour.[4]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /fəˈmɒtɪdiːn/ |
| Trade names | Pepcid, Zantac 360, others |
| AHFS/Drugs | Monograph |
| MedlinePlus | a687011 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth,intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokineticdata | |
| Bioavailability | 40–45% (by mouth)[2] |
| Protein binding | 15–20%[2] |
| Eliminationhalf-life | 2.5–3.5 hours[2] |
| Excretion | Kidney(25–30% unchanged [Oral])[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.116.793 |
| Chemical and physical data | |
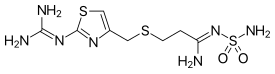
| Formula | C8H15N7O2S3 |
| Molar mass | 337.44g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Common side effects include headache, intestinal upset, and dizziness.[4]Serious side effects may includepneumoniaandseizures.[4][5]Use inpregnancyappears safe but has not been well studied, while use duringbreastfeedingis not recommended.[1]
Famotidine was patented in 1979 and came into medical use in 1985.[6]It is available as ageneric medication.[5]In 2022, it was the 49th most commonly prescribed medication in the United States, with more than 13million prescriptions.[7][8]
Medical uses
edit- heartburn,acid indigestion, and sour stomach
- Treatment forgastricandduodenal ulcers
- Treatment for pathologic gastrointestinal hypersecretory conditions such asZollinger–Ellison syndromeandmultiple endocrine adenomas
- Treatment forgastroesophageal reflux disease(GERD)
- Treatment foresophagitis
- Part of a multidrug regimen forHelicobacter pylorieradication, althoughomeprazolemay be somewhat more effective.[9][10][11][12][13][14]
- Prevention ofNSAID-induced peptic ulcers.[15][16]
- Given to surgery patients before operations to reduce the risk ofaspiration pneumonitis.[17][18][19]
Pharmacokinetics
editFamotidine has a delayed onset of action, beginning after 90 minutes. However, famotidine has a duration of effect of at least 540 minutes (9.0 h). At its peak effect, 210 minutes (3.5 h) after administration, famotidine reduces acid secretion by 7.3 mmol per 30 minutes.[20]
Side effects
editThe most common side effects associated with famotidine use includeheadache,dizziness,andconstipationordiarrhea.[21][22]
Famotidine may contribute toQT prolongation,[23]particularly when used with other QT-elongating drugs, or in people with poorkidney function.[24]
Mechanism of action
editActivation of H2receptors located onparietal cellsstimulatesproton pumpsto secrete acid into the stomach lumen. Famotidine, anH2antagonist,blocks the action ofhistamineon the parietal cells, ultimately reducing acid secretion into the stomach.
Interactions
editUnlikecimetidine,the first H2antagonist, famotidine has a minimal effect on thecytochrome P450enzyme system, and does not appear to interact with as many drugs as other medications in its class. Some exceptions include antiretrovirals such as atazanavir, chemotherapeutics such as doxorubicin, and antifungal medications such as itraconazole.[25][26][27]
History
editFamotidine was developed byYamanouchi PharmaceuticalCo.[28]It was licensed in the mid-1980s byMerck & Co.[29]and is marketed by a joint venture between Merck and Johnson & Johnson. Theimidazolering of cimetidine was replaced with a 2-guanidinothiazole ring. Famotidine proved to be nine times more potent thanranitidine,and thirty-two times more potent thancimetidine.[30]
It was first marketed in 1981. Pepcid RPDorally disintegrating tabletswere released in 1999. Generic preparations became available in 2001, e.g. Fluxid (Schwarz) or Quamatel (Gedeon Richter Ltd.).
In the United States and Canada, a product calledPepcid Complete,which combines famotidine with anantacidin a chewable tablet to quickly relieve the symptoms of excess stomach acid, is available. In the UK, this product was known as PepcidTwo until its discontinuation in April 2015.[31]
Famotidine has poorbioavailibility(50%) due to low gastroretention time. Famotidine is less soluble at higher pH, and when used in combination with antacids gastroretention time is increased. This promotes local delivery of these drugs to receptors in the parietal cell membrane and increases bioavailibility. Researchers are developing tablet formulations that rely on other gastroretentive drug delivery systems such as floating tablets to further increase bioavailibility.[32]
Society and culture
editCertain preparations of famotidine are availableover the counter(OTC) in various countries. In the United States and Canada, 10 mg and 20 mg tablets, sometimes in combination with anantacid,[33][34]are available OTC. Larger doses still require a prescription.
Formulations of famotidine in combination withibuprofenwere marketed byHorizon Pharmaunder the trade name Duexis.[35]
Research
editCOVID-19
editAt the start of theCOVID-19 pandemic,some doctors observed that anecdotally some hospitalized patients in China may have had better outcomes on famotidine than other patients that were not taking famotidine. This led to hypotheses about use of famotidine in treatment of COVID-19.[36][37]Famotidine was considered a possible treatment for COVID-19 due to its potential anti-inflammatory effects. It was thought that famotidine could modify lung inflammation caused by coronaviruses. However, studies have shown that famotidine is not effective in reducing mortality or improving recovery in COVID-19 patients.[38]Famotidine primarily works by blocking the effects of histamine and has some potential mechanisms of action that may contribute to its anti-inflammatory properties, including the inhibition of the production of certain pro-inflammatory cytokines such asTNF- AlphaandIL-6.[39][40]Another hypothesis was that famotidine might activate thevagus nerveinflammatory reflex to attenuate cytokine storm.[40]Yet another hypothesis was that famotidine can reduce the activation ofmast cellsand the subsequent release ofinflammatory mediators,therefore acting as amast cell stabilizer.[41][39]However, while famotidine may have some anti-inflammatory effects, there is currently insufficient evidence to support its use for treating inflammation associated with COVID-19.[38]Therefore, it is not recommended for this purpose.[42]
Other
editSmall-scale studies have shown inconsistent and inconclusive evidence of efficacy in treatment-refractoryschizophrenia.[43]
Veterinary uses
editFamotidine is given to dogs and cats with acid reflux.[44]
References
edit- ^ab"Famotidine Pregnancy and Breastfeeding Warnings".Drugs.Archivedfrom the original on 16 December 2023.Retrieved3 March2019.
- ^abcde"Famotidine tablet".DailyMed.Archivedfrom the original on 31 March 2024.Retrieved6 March2021.
- ^"Zantac 360- famotidine tablet, film coated".DailyMed.17 May 2022.Archivedfrom the original on 6 July 2022.Retrieved6 July2022.
- ^abcdef"Famotidine Monograph for Professionals".Drugs.American Society of Health-System Pharmacists.Archivedfrom the original on 17 June 2019.Retrieved3 March2019.
- ^abBritish national formulary: BNF 76(76 ed.). Pharmaceutical Press. 2018. pp. 74–75.ISBN9780857113382.
- ^Fischer J, Ganellin CR (2006).Analogue-based Drug Discovery.John Wiley & Sons. p. 444.ISBN9783527607495.Archivedfrom the original on 29 July 2020.Retrieved7 May2020.
- ^"The Top 300 of 2022".ClinCalc.Archivedfrom the original on 30 August 2024.Retrieved30 August2024.
- ^"Famotidine Drug Usage Statistics, United States, 2013 - 2022".ClinCalc.Archivedfrom the original on 17 April 2020.Retrieved30 August2024.
- ^Kanayama S (January 1999). "[Proton-pump inhibitors versus H2-receptor antagonists in triple therapy for Helicobacter pylori eradication]".Nihon Rinsho. Japanese Journal of Clinical Medicine.57(1): 153–6.PMID10036954.
- ^Soga T, Matsuura M, Kodama Y, Fujita T, Sekimoto I, Nishimura K, et al. (August 1999). "Is a proton pump inhibitor necessary for the treatment of lower-grade reflux esophagitis?".Journal of Gastroenterology.34(4): 435–40.doi:10.1007/s005350050292.PMID10452673.S2CID22115962.
- ^Borody TJ, Andrews P, Fracchia G, Brandl S, Shortis NP, Bae H (October 1995)."Omeprazole enhances efficacy of triple therapy in eradicating Helicobacter pylori".Gut.37(4): 477–81.doi:10.1136/gut.37.4.477.PMC1382896.PMID7489931.
- ^Hu FL, Jia JC, Li YL, Yang GB (2003)."Comparison of H2-receptor antagonist- and proton-pump inhibitor-based triple regimens for the eradication of Helicobacter pylori in Chinese patients with gastritis or peptic ulcer".The Journal of International Medical Research.31(6): 469–74.doi:10.1177/147323000303100601.PMID14708410.S2CID25818901.
- ^Kirika NV, Bodrug NI, Butorov IV, Butorov SI (2004). "[Efficacy of different schemes of anti-helicobacter therapy in duodenal ulcer]".Terapevticheskii Arkhiv.76(2): 18–22.PMID15106408.
- ^Fujiwara Y, Higuchi K, Nebiki H, Chono S, Uno H, Kitada K, et al. (June 2005)."Famotidine vs. omeprazole: a prospective randomized multicentre trial to determine efficacy in non-erosive gastro-oesophageal reflux disease".Alimentary Pharmacology & Therapeutics.21(Suppl 2): 10–8.doi:10.1111/j.1365-2036.2005.02468.x.PMID15943841.S2CID24690061.
- ^La Corte R, Caselli M, Castellino G, Bajocchi G, Trotta F (June 1999). "Prophylaxis and treatment of NSAID-induced gastroduodenal disorders".Drug Safety.20(6): 527–43.doi:10.2165/00002018-199920060-00006.PMID10392669.S2CID41990751.
- ^Laine L, Kivitz AJ, Bello AE, Grahn AY, Schiff MH, Taha AS (March 2012)."Double-blind randomized trials of single-tablet ibuprofen/high-dose famotidine vs. ibuprofen alone for reduction of gastric and duodenal ulcers".The American Journal of Gastroenterology.107(3): 379–86.doi:10.1038/ajg.2011.443.PMC3321505.PMID22186979.
- ^Escolano F, Castaño J, López R, Bisbe E, Alcón A (October 1992)."Effects of omeprazole, ranitidine, famotidine and placebo on gastric secretion in patients undergoing elective surgery".British Journal of Anaesthesia.69(4): 404–6.doi:10.1093/bja/69.4.404.PMID1419452.
- ^Vila P, Vallès J, Canet J, Melero A, Vidal F (November 1991)."Acid aspiration prophylaxis in morbidly obese patients: famotidine vs. ranitidine".Anaesthesia.46(11): 967–9.doi:10.1111/j.1365-2044.1991.tb09860.x.PMID1750602.
- ^Jahr JS, Burckart G, Smith SS, Shapiro J, Cook DR (July 1991). "Effects of famotidine on gastric pH and residual volume in pediatric surgery".Acta Anaesthesiologica Scandinavica.35(5): 457–60.doi:10.1111/j.1399-6576.1991.tb03328.x.PMID1887750.S2CID44356956.
- ^Feldman M (May 1996). "Comparison of the effects of over-the-counter famotidine and calcium carbonate antacid on postprandial gastric acid. A randomized controlled trial".JAMA.275(18): 1428–1431.doi:10.1001/jama.1996.03530420056036.PMID8618369.
- ^"Common Side Effects of Pepcid (Famotidine) Drug Center".RxList.Archivedfrom the original on 6 March 2019.Retrieved2 March2019.
- ^"Drugs & Medications".webmd.Archivedfrom the original on 6 March 2019.Retrieved2 March2019.
- ^Fazio G, Vernuccio F, Grutta G, Re GL (26 April 2013)."Drugs to be avoided in patients with long QT syndrome: Focus on the anaesthesiological management".World Journal of Cardiology.5(4): 87–93.doi:10.4330/wjc.v5.i4.87.PMC3653016.PMID23675554.
- ^Lee KW, Kayser SR, Hongo RH, Tseng ZH, Scheinman MM (May 2004). "Famotidine and long QT syndrome".The American Journal of Cardiology.93(10): 1325–1327.doi:10.1016/j.amjcard.2004.02.025.PMID15135720.
- ^Wang X, Boffito M, Zhang J, Chung E, Zhu L, Wu Y, et al. (September 2011)."Effects of the H2-receptor antagonist famotidine on the pharmacokinetics of atazanavir-ritonavir with or without tenofovir in HIV-infected patients".AIDS Patient Care and STDs.25(9): 509–515.doi:10.1089/apc.2011.0113.PMC3157302.PMID21770762.
- ^Hegazy SK, El-Haggar SM, Alhassanin SA, El-Berri EI (January 2021). "Comparative randomized trial evaluating the effect of proton pump inhibitor versus histamine 2 receptor antagonist as an adjuvant therapy in diffuse large B-cell lymphoma".Medical Oncology.38(1): 4.doi:10.1007/s12032-020-01452-z.PMID33394214.S2CID230485193.
- ^Lim SG, Sawyerr AM, Hudson M, Sercombe J, Pounder RE (June 1993). "Short report: the absorption of fluconazole and itraconazole under conditions of low intragastric acidity".Alimentary Pharmacology & Therapeutics.7(3): 317–321.doi:10.1111/j.1365-2036.1993.tb00103.x.PMID8117350.S2CID20462864.
- ^US patent 4283408,Yasufumi Hirata, Isao Yanagisawa, Yoshio Ishii, Shinichi Tsukamoto, Noriki Ito, Yasuo Isomura and Masaaki Takeda, "Guanidinothiazole compounds, process for preparation and gastric inhibiting compositions containing them", issued 11 August 1981
- ^"Sankyo Pharma".Skyscape Mediwire. 2002. Archived fromthe originalon 23 February 2009.Retrieved31 October2009.
- ^Howard JM, Chremos AN, Collen MJ, McArthur KE, Cherner JA, Maton PN, et al. (April 1985)."Famotidine, a new, potent, long-acting histamine H2-receptor antagonist: comparison with cimetidine and ranitidine in the treatment of Zollinger-Ellison syndrome".Gastroenterology.88(4): 1026–33.doi:10.1016/s0016-5085(85)80024-x.PMID2857672.
- ^"PepcidTwo Chewable Tablet".Archived fromthe originalon 18 July 2016.Retrieved7 June2015.
- ^"Formulation and Evaluation of Gastroretentive Floating Tablets of Famotidine".Farmavita.Net. 2008. Archived fromthe originalon 29 March 2016.Retrieved31 January2009.
- ^Pepcid Complete
- ^"Famotidine".Medline Plus.Archivedfrom the original on 4 October 2022.Retrieved21 March2018.
- ^"Duexis".Drugs.Archivedfrom the original on 26 November 2020.Retrieved28 April2020.
- ^Borrell B (April 2020)."New York clinical trial quietly tests heartburn remedy against coronavirus".Science.doi:10.1126/science.abc4739.Archivedfrom the original on 29 April 2020.Retrieved20 September2022.
- ^Malone RW, Tisdall P, Fremont-Smith P, Liu Y, Huang XP, White KM, et al. (2021)."COVID-19: Famotidine, Histamine, Mast Cells, and Mechanisms".Frontiers in Pharmacology.12:633680.doi:10.3389/fphar.2021.633680.PMC8021898.PMID33833683.
- ^abCheema HA, Shafiee A, Athar M, Shahid A, Awan RU, Afifi AM, et al. (February 2023)."No evidence of clinical efficacy of famotidine for the treatment of COVID-19: a systematic review and meta-analysis".J Infect.86(2): 154–225.doi:10.1016/j.jinf.2022.11.022.PMC9711899.PMID36462586.
- ^abKow CS, Ramachandram DS, Hasan SS (September 2023)."Famotidine: A potential mitigator of mast cell activation in post-COVID-19 cognitive impairment".J Psychosom Res.172:111425.doi:10.1016/j.jpsychores.2023.111425.PMC10292911.PMID37399740.
- ^abYang H, George SJ, Thompson DA, Silverman HA, Tsaava T, Tynan A, et al. (May 2022)."Famotidine activates the vagus nerve inflammatory reflex to attenuate cytokine storm".Mol Med.28(1): 57.doi:10.1186/s10020-022-00483-8.PMC9109205.PMID35578169.
- ^Salvucci F, Codella R, Coppola A, Zacchei I, Grassi G, Anti ML, et al. (2023)."Antihistamines improve cardiovascular manifestations and other symptoms of long-COVID attributed to mast cell activation".Front Cardiovasc Med.10:1202696.doi:10.3389/fcvm.2023.1202696.PMC10388239.PMID37529714.
- ^Long B, Chavez S, Carius BM, Brady WJ, Liang SY, Koyfman A, et al. (June 2022)."Clinical update on COVID-19 for the emergency and critical care clinician: Medical management".The American Journal of Emergency Medicine(Review).56:158–170.doi:10.1016/j.ajem.2022.03.036.PMC8956349.PMID35397357.
- ^Andrade C (2013)."Famotidine Augmentation in Schizophrenia: Hope or Hype?".The Journal of Clinical Psychiatry.74(9): e855–e858.doi:10.4088/JCP.13f08707.PMID24107771.Archivedfrom the original on 11 January 2024.Retrieved11 January2024.
- ^"Famotidine".PetMD.Archivedfrom the original on 19 May 2015.Retrieved7 June2015.
External links
editMedia related toFamotidineat Wikimedia Commons