Gamma secretaseis a multi-subunitproteasecomplex, anintegral membrane protein,that cleaves single-passtransmembrane proteinsat residues within the transmembrane domain. Proteases of this type are known asintramembrane proteases.The most well-known substrate of gamma secretase isamyloid precursor protein,a large integral membrane protein that, when cleaved by both gamma andbeta secretase,produces a short 37-43[verification needed]amino acidpeptidecalledamyloid betawhose abnormallyfoldedfibrillar form is the primary component ofamyloid plaquesfound in the brains ofAlzheimer's diseasepatients. Gamma secretase is also critical in the related processing of several other type I integral membrane proteins, such asNotch,[2]ErbB4,[3]E-cadherin,[4]N-cadherin,[5]ephrin-B2,[6]orCD44.[7]
| Gamma-secretase (Nicastrin subunit) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
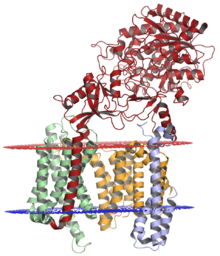 The gamma secretase complex, withnicastrin(red),presenilin-1(orange),PEN-2(blue), andAPH-1(green); lumenal membrane shown in red and cytoplasmic membrane shown in blue. The structure was solved bycryo-electron microscopy.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Gamma-secretase, γ-secretase | ||||||||
| Pfam | PF05450 | ||||||||
| InterPro | IPR008710 | ||||||||
| OPM superfamily | 244 | ||||||||
| OPM protein | [ 5fn5[ | ||||||||
| Membranome | 155 | ||||||||
| |||||||||
Subunits and assembly
editThe gamma secretase complex consists of four individual proteins:PSEN1(presenilin-1),[8]nicastrin,APH-1(anterior pharynx-defective 1), andPEN-2(presenilin enhancer 2).[9]Recent evidence suggests that a fifth protein, known asCD147,is a non-essential regulator of the complex whose absence increases activity.[10][11]Presenilin,anaspartyl protease,is thecatalyticsubunit; mutations in the presenilin gene a majorgeneticrisk factor for Alzheimer's disease[12]and modulates immune cell activity.[13]In humans, two forms of presenilin and two forms of APH-1 have been identified in thegenome;one of the APHhomologscan also be expressed in two isoforms viaalternative splicing,leading to at least six different possible gamma secretase complexes that may have tissue- or cell type specificity.[14]
The proteins in the gamma secretase complex are heavily modified byproteolysisduring assembly and maturation of the complex; a required activation step is in the autocatalytic cleavage of presenilin to N- and C-terminal fragments. Nicastrin's primary role is in maintaining the stability of the assembled complex and regulating intracellular protein trafficking.[15]PEN-2 associates with the complex via binding of a transmembrane domain of presenilin[16]and, among other possible roles, helps to stabilize the complex after presenilin proteolysis has generated the activatedN-terminalandC-terminalfragments.[17]APH-1, which is required for proteolytic activity, binds to the complex via aconservedAlpha helixinteractionmotifand aids in initiating assembly of premature components.[18]
Recent research has shown that the interaction of the gamma secretase complex with theγ-secretase activating proteinfacilitates the gamma cleavage ofamyloid precursor proteinintoβ-amyloid.[19]
Cellular trafficking
editThe gamma secretase complex is thought to assemble and mature via proteolysis in the earlyendoplasmic reticulum.[20]The complexes are then transported to the late ER where they interact with and cleave their substrate proteins.[21]Gamma secretase complexes have also been observed localized to themitochondria,where they may play a role in promotingapoptosis.[22]
Function
editGamma secretase is an internal protease that cleaves within the membrane-spanning domain of itssubstrateproteins, includingamyloid precursor protein(APP) andNotch.Substrate recognition occurs via nicastrin ectodomain binding to the N-terminus of the target, which is then passed via a poorly understood process between the two presenilin fragments to awater-containingactive sitewhere the catalyticaspartateresidue is located. The active site must contain water to carry outhydrolysiswithin ahydrophobicenvironment in the interior of thecell membrane,although it is not well understood how water andprotonexchange is effected, and as yet noX-ray crystallographystructure of gamma secretase is available.[23]Low-resolutionelectron microscopyreconstructions have allowed the visualization of the hypothesized internal pores of about 2 nanometres.[24]In 2014, a three-dimensional structure of an intact human gamma-secretase complex was determined bycryo-electron microscopysingle-particle analysis at 4.5 angstrom resolution[25]and in 2015 an atomic-resolution (3.4 angstrom) cryo-EM structure was reported.[1]
The gamma secretase complex is unusual among proteases in having a "sloppy" cleavage site at the C-terminal site inamyloid betageneration; gamma secretase can cleave APP in any of multiple sites to generate a peptide of variable length, most typically from 39 to 42 amino acids long, with Aβ40 the most common isoform and Aβ42 the most susceptible toconformational changesleading toamyloidfibrillogenesis. Certain mutations in both APP and both types of human presenilin are associated with increased Aβ42 production and the early-onset genetic form offamilial Alzheimer's disease.[26]Although older data suggested that different forms of the gamma secretase complex could be differentially responsible for generating different amyloid beta isoforms,[27]current evidence indicates that the C-terminus of amyloid beta is produced by a series of single-residue cleavages by the same gamma secretase complex.[28][29][30]Earlier cleavage sites produce peptides of length 46 (zeta-cleavage) and 49 (epsilon-cleavage).[29]
See also
edit- DAPT (chemical),a γ-secretase inhibitor
- Nirogacestat,a γ-secretase inhibitor
- Semagacestat,a γ-secretase inhibitor
References
edit- ^abBai, Xiao-chen; Yan, Chuangye; Yang, Guanghui; Lu, Peilong; Ma, Dan; Sun, Linfeng; Zhou, Rui;Scheres, Sjors H. W.;Shi, Yigong (17 August 2015)."An atomic structure of human γ-secretase".Nature.525(7568): 212–217.doi:10.1038/nature14892.PMC4568306.PMID26280335.
- ^De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R (1999). "A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain".Nature.398(6727): 518–22.doi:10.1038/19083.PMID10206645.S2CID4346474.
- ^Ni CY, Murphy MP, Golde TE, Carpenter G (2001). "gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase".Science.294(5549): 2179–81.doi:10.1126/science.1065412.PMID11679632.S2CID23227013.
- ^Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK (2002)."A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions".EMBO J.21(8): 1948–56.doi:10.1093/emboj/21.8.1948.PMC125968.PMID11953314.
- ^Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK (2003)."A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations".Cell.114(5): 635–45.doi:10.1016/j.cell.2003.08.008.PMID13678586.S2CID7265454.
- ^Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK (2006)."Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling".EMBO J.25(6): 1242–52.doi:10.1038/sj.emboj.7601031.PMC1422162.PMID16511561.
- ^Lammich S, Okochi M, Takeda M, Kaether C, Capell A, Zimmer AK, Edbauer D, Walter J, Steiner H, Haass C (2002)."Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Abeta-like peptide".J Biol Chem.277(47): 44754–9.doi:10.1074/jbc.M206872200.PMID12223485.
- ^Sobhanifar, S; Schneider, B; Löhr, F; Gottstein, D; Ikeya, T; Mlynarczyk, K; Pulawski, W; Ghoshdastider, U; Kolinski, M; Filipek, S; Güntert, P; Bernhard, F; Dötsch, V (25 May 2010)."Structural investigation of the C-terminal catalytic fragment of presenilin 1".Proceedings of the National Academy of Sciences of the United States of America.107(21): 9644–9.doi:10.1073/pnas.1000778107.PMC2906861.PMID20445084.
- ^Kaether C, Haass C, Steiner H (2006)."Assembly, trafficking and function of gamma-secretase"(PDF).Neurodegener Dis.3(4–5): 275–83.doi:10.1159/000095267.PMID17047368.S2CID17324271.
- ^Zhou S, Zhou H, Walian PJ, Jap BK (April 2006). "The discovery and role of CD147 as a subunit of gamma-secretase complex".Drug News Perspect.19(3): 133–8.doi:10.1358/dnp.2006.19.3.985932.PMID16804564.
- ^Zhou S, Zhou H, Walian PJ, Jap BK (May 2005)."CD147 is a regulatory subunit of the γ-secretase complex in Alzheimer's disease amyloid β-peptide production".Proc. Natl. Acad. Sci. U.S.A.102(21): 7499–504.doi:10.1073/pnas.0502768102.PMC1103709.PMID15890777.
- ^Chen F, Hasegawa H, Schmitt-Ulms G, Kawarai T, Bohm C, Katayama T, Gu Y, Sanjo N, Glista M, Rogaeva E, Wakutani Y, Pardossi-Piquard R, Ruan X, Tandon A, Checler F, Marambaud P, Hansen K, Westaway D, St George-Hyslop P, Fraser P (April 2006). "TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity".Nature.440(7088): 1208–12.doi:10.1038/nature04667.PMID16641999.S2CID4349251.
- ^Farfara D, Trudler D, Segev-Amzaled N, Galron R, Stein R, Frenkel D (November 2010). "g secretase component presenilin is important for microglia b-Amyloid clearance".Annals of Neurology.69(1): 170–80.doi:10.1002/ana.22191.PMID21280087.S2CID20603724.
- ^Shirotani K, Edbauer D, Prokop S, Haass C, Steiner H (2004)."Identification of distinct gamma-secretase complexes with different APH-1 variants".J Biol Chem.279(40): 41340–5.doi:10.1074/jbc.M405768200.PMID15286082.
- ^Zhang YW, Luo WJ, Wang H, Lin P, Vetrivel KS, Liao F, Li F, Wong PC, Farquhar MG, Thinakaran G, Xu H (April 2005)."Nicastrin Is Critical for Stability and Trafficking but Not Association of Other Presenilin/γ-Secretase Components".J. Biol. Chem.280(17): 17020–6.doi:10.1074/jbc.M409467200.PMC1201533.PMID15711015.
- ^Watanabe N, Tomita T, Sato C, Kitamura T, Morohashi Y, Iwatsubo T (December 2005)."Pen-2 is incorporated into the gamma-secretase complex through binding to transmembrane domain 4 of presenilin 1".J. Biol. Chem.280(51): 41967–75.doi:10.1074/jbc.M509066200.PMID16234244.
- ^Prokop S, Shirotani K, Edbauer D, Haass C, Steiner H (May 2004)."Requirement of PEN-2 for stabilization of the presenilin N-/C-terminal fragment heterodimer within the gamma-secretase complex".J. Biol. Chem.279(22): 23255–61.doi:10.1074/jbc.M401789200.PMID15039426.
- ^Lee SF, Shah S, Yu C, Wigley WC, Li H, Lim M, Pedersen K, Han W, Thomas P, Lundkvist J, Hao YH, Yu G (February 2004)."A conserved GXXXG motif in APH-1 is critical for assembly and activity of the gamma-secretase complex".J. Biol. Chem.279(6): 4144–52.doi:10.1074/jbc.M309745200.PMID14627705.
- ^He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, Greengard P (September 2010)."Gamma-secretase activating protein, a therapeutic target for Alzheimer's disease".Nature.467(2): 95–98.doi:10.1038/nature09325.PMC2936959.PMID20811458.
- Gina Kolata (September 1, 2010)."Finding Suggests New Aim for Alzheimer's Drugs".The New York Times.
- ^Capell A, Beher D, Prokop S, Steiner H, Kaether C, Shearman MS, Haass C (February 2005)."Gamma-secretase complex assembly within the early secretory pathway".J. Biol. Chem.280(8): 6471–8.doi:10.1074/jbc.M409106200.PMID15591316.
- ^Kim SH, Yin YI, Li YM, Sisodia SS (November 2004)."Evidence that assembly of an active gamma-secretase complex occurs in the early compartments of the secretory pathway".J. Biol. Chem.279(47): 48615–9.doi:10.1074/jbc.C400396200.PMID15456788.
- ^Hansson CA, Frykman S, Farmery MR, Tjernberg LO, Nilsberth C, Pursglove SE, Ito A, Winblad B, Cowburn RF, Thyberg J, Ankarcrona M (December 2004)."Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria".J. Biol. Chem.279(49): 51654–60.doi:10.1074/jbc.M404500200.PMID15456764.
- ^Wolfe MS (July 2006). "The gamma-secretase complex: membrane-embedded proteolytic ensemble".Biochemistry.45(26): 7931–9.doi:10.1021/bi060799c.PMID16800619.
- ^Lazarov VK, Fraering PC, Ye W, Wolfe MS, Selkoe DJ, Li H (May 2006)."Electron microscopic structure of purified, active γ-secretase reveals an aqueous intramembrane chamber and two pores".Proc. Natl. Acad. Sci. U.S.A.103(18): 6889–94.doi:10.1073/pnas.0602321103.PMC1458989.PMID16636269.
- ^Lu P, Bai XC, Ma D, Xie T, Yan C, Sun L, Yang G, Zhao Y, Zhou R, Scheres SH, Shi Y (August 2014)."Three-dimensional structure of human γ-secretase".Nature.512(7513): 166–170.doi:10.1038/nature13567.PMC4134323.PMID25043039.
- ^Wiley JC, Hudson M, Kanning KC, Schecterson LC, Bothwell M (September 2005)."Familial Alzheimer's disease mutations inhibit gamma-secretase-mediated liberation of beta-amyloid precursor protein carboxy-terminal fragment".J. Neurochem.94(5): 1189–201.doi:10.1111/j.1471-4159.2005.03266.x.PMID15992373.
- ^Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR (January 2004)."Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase".Hum. Mol. Genet.13(2): 159–70.doi:10.1093/hmg/ddh019.PMID14645205.
- ^Zhao G, Tan J, Mao G, Cui MZ, Xu X (March 2007)."The same gamma-secretase accounts for the multiple intramembrane cleavages of APP".J. Neurochem.100(5): 1234–46.doi:10.1111/j.1471-4159.2006.04302.x.PMID17241131.
- ^abZhang, H; Ma, Q; Zhang, YW; Xu, H (January 2012)."Proteolytic processing of Alzheimer's β-amyloid precursor protein".Journal of Neurochemistry.120 Suppl 1: 9–21.doi:10.1111/j.1471-4159.2011.07519.x.PMC3254787.PMID22122372.
- ^Haass, C; Kaether, C; Thinakaran, G; Sisodia, S (May 2012)."Trafficking and proteolytic processing of APP".Cold Spring Harbor Perspectives in Medicine.2(5): a006270.doi:10.1101/cshperspect.a006270.PMC3331683.PMID22553493.