Ammoniais aninorganicchemical compoundofnitrogenandhydrogenwith theformulaNH3.Astable binary hydrideand the simplestpnictogen hydride,ammonia is a colourlessgaswith a distinctive pungent smell. Biologically, it is a commonnitrogenous waste,and it contributes significantly to thenutritionalneeds of terrestrial organisms by serving as a precursor tofertilisers.[13]Around 70% of ammonia produced industrially is used to make fertilisers[14]in various forms and composition, such asureaanddiammonium phosphate.Ammonia in pure form is also applied directly into the soil.
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ammonia[1]
| |||
| Systematic IUPAC name
Azane | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 3587154 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.760 | ||
| EC Number |
| ||
| 79 | |||
| KEGG | |||
| MeSH | Ammonia | ||
PubChemCID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1005 | ||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| NH3 | |||
| Molar mass | 17.031g·mol−1 | ||
| Appearance | Colourless gas | ||
| Odor | Strong pungent odour | ||
| Density |
| ||
| Melting point | −77.73 °C (−107.91 °F; 195.42 K) (Triple pointat 6.060 kPa, 195.4 K) | ||
| Boiling point | −33.34 °C (−28.01 °F; 239.81 K) | ||
| Critical point(T,P) | 132.4 °C (405.5 K), 111.3 atm (11,280 kPa) | ||
| |||
| Solubility | soluble inchloroform,ether,ethanol,methanol | ||
| Vapor pressure | 857.3 kPa | ||
| Acidity(pKa) | 32.5 (−33 °C),[6]9.24 (of ammonium) | ||
| Basicity(pKb) | 4.75 | ||
| Conjugate acid | Ammonium | ||
| Conjugate base | Amide | ||
| −18.0×10−6cm3/mol | |||
Refractive index(nD)
|
1.3327 | ||
| Viscosity |
| ||
| Structure | |||
| C3v | |||
| Trigonal pyramid | |||
| 1.42D | |||
| Thermochemistry | |||
Std molar
entropy(S⦵298) |
193 J/(mol·K)[8] | ||
Std enthalpy of
formation(ΔfH⦵298) |
−46 kJ/mol[8] | ||
| Hazards | |||
| GHSlabelling:[11] | |||
  
| |||
| Danger | |||
| H314,H331,H410 | |||
| P260,P273,P280,P303+P361+P353,P304+P340+P311,P305+P351+P338+P310 | |||
| NFPA 704(fire diamond) | |||
| 651 °C (1,204 °F; 924 K) | |||
| Explosive limits | 15.0–33.6% | ||
| Lethal doseor concentration (LD, LC): | |||
LD50(median dose)
|
350 mg/kg (rat, oral)[9] | ||
LC50(median concentration)
|
| ||
LCLo(lowest published)
|
| ||
| NIOSH(US health exposure limits):[12] | |||
PEL(Permissible)
|
50 ppm (25 ppmACGIH-TLV; 35 ppmSTEL) | ||
REL(Recommended)
|
TWA 25 ppm (18 mg/m3) ST 35 ppm (27 mg/m3) | ||
IDLH(Immediate danger)
|
300 ppm | ||
| Safety data sheet(SDS) | ICSC 0414(anhydrous) | ||
| Related compounds | |||
Related nitrogen hydrides
|
|||
Related compounds
|
|||
| Supplementary data page | |||
| Ammonia (data page) | |||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
Ammonia, either directly or indirectly, is also a building block for the synthesis of many chemicals.
Ammonia occurs in nature and has been detected in the interstellar medium. In many countries, it is classified as anextremely hazardous substance.[15]
Ammonia is produced biologically in a process callednitrogen fixation,but even more is generated industrially by theHaber process.The process helped revolutionize agriculture by providing cheap fertilizers. The global industrial production of ammonia in 2021 was 235 million tonnes.[16][17]Industrial ammonia is transported by road intankers,by rail intank wagons,by sea ingas carriers,or incylinders.[18]
Ammonia boils at −33.34 °C (−28.012 °F) at a pressure of oneatmosphere,but the liquid can often be handled in the laboratory without external cooling. Household ammonia orammonium hydroxideis a solution of ammonia in water.
Etymology
editPliny,in Book XXXI of hisNatural History,refers to a salt namedhammoniacum,so called because of the proximity of its source to the Temple ofJupiter Amun(GreekἌμμωνAmmon) in the Roman province ofCyrenaica.[19]However, the description Pliny gives of the salt does not conform to the properties ofammonium chloride.According toHerbert Hoover's commentary in his English translation ofGeorgius Agricola'sDe re metallica,it is likely to have been common sea salt.[20]In any case, that salt ultimately gave ammonia andammoniumcompounds their name.
Natural occurrence (abiological)
editTraces of ammonia/ammonium are found in rainwater.Ammonium chloride(sal ammoniac), andammonium sulfateare found in volcanic districts. Crystals ofammonium bicarbonatehave been found inPatagoniaguano.[21]
Ammonia is found throughout theSolar SystemonMars,Jupiter,Saturn,Uranus,Neptune,andPluto,among other places: on smaller, icybodiessuch as Pluto, ammonia can act as a geologically important antifreeze, as a mixture of water and ammonia can have a melting point as low as −100 °C (−148 °F; 173 K) if the ammonia concentration is high enough and thus allow such bodies to retain internal oceans and active geology at a far lower temperature than would be possible with water alone.[22][23]Substances containing ammonia, or those that are similar to it, are calledammoniacal.[24]
Properties
editAmmonia is a colourlessgaswith a characteristicallypungent smell.It islighter than air,its density being 0.589 times that ofair.It is easily liquefied due to the stronghydrogen bondingbetween molecules. Gaseous ammonia turns to a colourlessliquid,whichboilsat −33.1 °C (−27.58 °F), andfreezesto colourless crystals[21]at −77.7 °C (−107.86 °F). Little data is available at very high temperatures and pressures, but theliquid-vapor critical pointoccurs at 405 K and 11.35 MPa.[25]
Solid
editThe crystal symmetry is cubic,Pearson symbolcP16,space groupP213 No.198, lattice constant 0.5125nm.[26]
Liquid
editLiquidammonia possesses strongionisingpowers reflecting its highεof 22 at −35 °C (−31 °F).[27]Liquid ammonia has a very highstandard enthalpy change of vapourization(23.5kJ/mol;[28]for comparison,water's is 40.65 kJ/mol, methane 8.19 kJ/mol andphosphine14.6 kJ/mol) and can be transported in pressurized or refrigerated vessels; however, atstandard temperature and pressureliquid anhydrous ammonia will vaporize.[29]
Solvent properties
editAmmonia readilydissolvesin water. In an aqueous solution, it can be expelled by boiling. Theaqueoussolution of ammonia isbasic,and may be described as aqueous ammonia orammonium hydroxide.[30]The maximum concentration of ammonia in water (asaturated solution) has aspecific gravityof 0.880 and is often known as '.880 ammonia'.[31]
| Temperature (°C) |
Density (kg/m3) |
Specific heat (kJ/(kg·K)) |
Kinematic viscosity (m2/s) |
Thermal conductivity (W/(m·K)) |
Thermal diffusivity (m2/s) |
Prandtl Number |
Bulk modulus (K−1) |
|---|---|---|---|---|---|---|---|
| −50 | 703.69 | 4.463 | 4.35×10−7 | 0.547 | 1.74×10−7 | 2.6 | |
| −40 | 691.68 | 4.467 | 4.06×10−7 | 0.547 | 1.78×10−7 | 2.28 | |
| −30 | 679.34 | 4.476 | 3.87×10−7 | 0.549 | 1.80×10−7 | 2.15 | |
| −20 | 666.69 | 4.509 | 3.81×10−7 | 0.547 | 1.82×10−7 | 2.09 | |
| −10 | 653.55 | 4.564 | 3.78×10−7 | 0.543 | 1.83×10−7 | 2.07 | |
| 0 | 640.1 | 4.635 | 3.73×10−7 | 0.540 | 1.82×10−7 | 2.05 | |
| 10 | 626.16 | 4.714 | 3.68×10−7 | 0.531 | 1.80×10−7 | 2.04 | |
| 20 | 611.75 | 4.798 | 3.59×10−7 | 0.521 | 1.78×10−7 | 2.02 | 2.45×10−3 |
| 30 | 596.37 | 4.89 | 3.49×10−7 | 0.507 | 1.74×10−7 | 2.01 | |
| 40 | 580.99 | 4.999 | 3.40×10−7 | 0.493 | 1.70×10−7 | 2 | |
| 50 | 564.33 | 5.116 | 3.30×10−7 | 0.476 | 1.65×10−7 | 1.99 |
| Temperature (K) |
Temperature (°C) | Density (kg/m3) |
Specific heat (kJ/(kg·K)) |
Dynamic viscosity (kg/(m·s)) |
Kinematic viscosity (m2/s) |
Thermal conductivity (W/(m·K)) |
Thermal diffusivity (m2/s) |
Prandtl Number |
|---|---|---|---|---|---|---|---|---|
| 273 | -0.15 | 0.7929 | 2.177 | 9.35×10−6 | 1.18×10−5 | 0.0220 | 1.31×10−5 | 0.90 |
| 323 | 49.85 | 0.6487 | 2.177 | 1.10×10−5 | 1.70×10−5 | 0.0270 | 1.92×10−5 | 0.88 |
| 373 | 99.85 | 0.559 | 2.236 | 1.29×10−5 | 1.30×10−5 | 0.0327 | 2.62×10−5 | 0.87 |
| 423 | 149.85 | 0.4934 | 2.315 | 1.47×10−5 | 2.97×10−5 | 0.0391 | 3.43×10−5 | 0.87 |
| 473 | 199.85 | 0.4405 | 2.395 | 1.65×10−5 | 3.74×10−5 | 0.0467 | 4.42×10−5 | 0.84 |
| 480 | 206.85 | 0.4273 | 2.43 | 1.67×10−5 | 3.90×10−5 | 0.0492 | 4.74×10−5 | 0.822 |
| 500 | 226.85 | 0.4101 | 2.467 | 1.73×10−5 | 4.22×10−5 | 0.0525 | 5.19×10−5 | 0.813 |
| 520 | 246.85 | 0.3942 | 2.504 | 1.80×10−5 | 4.57×10−5 | 0.0545 | 5.52×10−5 | 0.827 |
| 540 | 266.85 | 0.3795 | 2.54 | 1.87×10−5 | 4.91×10−5 | 0.0575 | 5.97×10−5 | 0.824 |
| 560 | 286.85 | 0.3708 | 2.577 | 1.93×10−5 | 5.20×10−5 | 0.0606 | 6.34×10−5 | 0.827 |
| 580 | 306.85 | 0.3533 | 2.613 | 2.00×10−5 | 5.65×10−5 | 0.0638 | 6.91×10−5 | 0.817 |
Liquid ammonia is a widely studied nonaqueous ionising solvent. Its most conspicuous property is its ability to dissolve alkali metals to form highly coloured, electrically conductive solutions containingsolvated electrons.Apart from these remarkable solutions, much of the chemistry in liquid ammonia can be classified by analogy with related reactions inaqueous solutions.Comparison of the physical properties ofNH3with those of water showsNH3has the lower melting point, boiling point, density,viscosity,dielectric constantandelectrical conductivity.These differences are attributed at least in part to the weaker hydrogen bonding inNH3.The ionic self-dissociation constantof liquidNH3at −50 °C is about 10−33.
| Solubility (g of salt per 100 g liquidNH3) | |
|---|---|
| Ammonium acetate | 253.2 |
| Ammonium nitrate | 389.6 |
| Lithium nitrate | 243.7 |
| Sodium nitrate | 97.6 |
| Potassium nitrate | 10.4 |
| Sodium fluoride | 0.35 |
| Sodium chloride | 157.0 |
| Sodium bromide | 138.0 |
| Sodium iodide | 161.9 |
| Sodium thiocyanate | 205.5 |
Liquid ammonia is an ionising solvent, although less so than water, and dissolves a range of ionic compounds, including manynitrates,nitrites,cyanides,thiocyanates,metal cyclopentadienyl complexesandmetal bis(trimethylsilyl)amides.[32]Most ammonium salts are soluble and act as acids in liquid ammonia solutions. The solubility ofhalidesalts increases fromfluoridetoiodide.A saturated solution ofammonium nitrate(Divers' solution,named afterEdward Divers) contains 0.83 mol solute per mole of ammonia and has avapour pressureof less than 1 bar even at 25 °C (77 °F). However, fewoxyanionsalts with other cations dissolve.[34]
Liquid ammonia will dissolve all of thealkali metalsand otherelectropositivemetals such asCa,[35]Sr,Ba,EuandYb(alsoMgusing an electrolytic process[33]). At low concentrations (<0.06 mol/L), deep blue solutions are formed: these contain metal cations andsolvated electrons,free electrons that are surrounded by a cage of ammonia molecules.
These solutions are strong reducing agents. At higher concentrations, the solutions are metallic in appearance and in electrical conductivity. At low temperatures, the two types of solution can coexist asimmisciblephases.
Redox properties of liquid ammonia
edit| E°(V, ammonia) | E°(V, water) | |
|---|---|---|
| Li++ e−⇌ Li | −2.24 | −3.04 |
| K++ e−⇌ K | −1.98 | −2.93 |
| Na++ e−⇌ Na | −1.85 | −2.71 |
| Zn2++ 2 e−⇌ Zn | −0.53 | −0.76 |
| 2 [NH4]++ 2 e−⇌ H2+ 2 NH3 | 0.00 | — |
| Cu2++ 2 e−⇌ Cu | +0.43 | +0.34 |
| Ag++ e−⇌ Ag | +0.83 | +0.80 |
The range of thermodynamic stability of liquid ammonia solutions is very narrow, as the potential for oxidation to dinitrogen,E°(N2+ 6 [NH4]++ 6 e−⇌ 8 NH3), is only +0.04 V. In practice, both oxidation to dinitrogen and reduction to dihydrogen are slow. This is particularly true of reducing solutions: the solutions of the alkali metals mentioned above are stable for several days, slowly decomposing to themetal amideand dihydrogen. Most studies involving liquid ammonia solutions are done in reducing conditions; although oxidation of liquid ammonia is usually slow, there is still a risk of explosion, particularly iftransition metalions are present as possible catalysts.
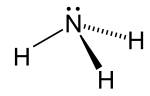
Structure
editThe ammonia molecule has atrigonal pyramidalshape, as predicted by thevalence shell electron pair repulsion theory(VSEPR theory) with an experimentally determined bond angle of 106.7°.[36]The central nitrogen atom has five outer electrons with an additional electron from each hydrogen atom. This gives a total of eight electrons, or four electron pairs that are arrangedtetrahedrally.Three of theseelectron pairsare used as bond pairs, which leaves onelone pairof electrons. The lone pair repels more strongly than bond pairs; therefore, the bond angle is not 109.5°, as expected for a regular tetrahedral arrangement, but 106.8°.[36]This shape gives the molecule adipolemoment and makes itpolar.The molecule's polarity, and especially its ability to formhydrogen bonds,makes ammonia highly miscible with water. The lone pair makes ammonia abase,a proton acceptor. Ammonia is moderately basic; a 1.0Maqueous solutionhas apHof 11.6, and if a strong acid is added to such a solution until the solution is neutral (pH = 7), 99.4% of the ammonia molecules areprotonated.Temperature andsalinityalso affect the proportion ofammonium[NH4]+.The latter has the shape of a regulartetrahedronand isisoelectronicwithmethane.
The ammonia molecule readily undergoesnitrogen inversionat room temperature; a useful analogy is anumbrellaturning itself inside out in a strong wind. Theenergy barrierto this inversion is 24.7 kJ/mol, and theresonance frequencyis 23.79GHz,corresponding tomicrowaveradiation of awavelengthof 1.260 cm. The absorption at this frequency was the firstmicrowave spectrumto be observed[37]and was used in the firstmaser.
Amphotericity
editOne of the most characteristic properties of ammonia is itsbasicity.Ammonia is considered to be a weak base. It combines withacidsto formammoniumsalts;thus, withhydrochloric acidit formsammonium chloride(sal ammoniac); withnitric acid,ammonium nitrate,etc. Perfectly dry ammonia gas will not combine with perfectly dryhydrogen chloridegas; moisture is necessary to bring about the reaction.[38][39]
As a demonstration experiment under air with ambient moisture, opened bottles of concentrated ammonia andhydrochloric acidsolutions produce a cloud ofammonium chloride,which seems to appear 'out of nothing' as the saltaerosolforms where the twodiffusingclouds of reagents meet between the two bottles.
- NH3+ HCl → [NH4]Cl
The salts produced by the action of ammonia on acids are known as theammonium saltsand all contain theammonium ion([NH4]+).[38]
Although ammonia is well known as a weak base, it can also act as an extremely weak acid. It is aprotic substanceand is capable of formation ofamides(which contain theNH−2ion). For example,lithiumdissolves inliquid ammoniato give a blue solution (solvated electron) oflithium amide:
- 2 Li + 2 NH3→ 2 LiNH2+ H2
Self-dissociation
editLike water, liquid ammonia undergoesmolecular autoionisationto form itsacid and base conjugates:
- 2 NH3⇌ NH+4+ NH−2
Ammonia often functions as aweak base,so it has somebufferingability. Shifts in pH will cause more or fewerammoniumcations (NH+4) andamide anions(NH−2) to be present insolution.At standard pressure and temperature,
- K =[NH+4] × [NH−2]= 10−30.
Combustion
editAmmonia does not burn readily or sustaincombustion,except under narrow fuel-to-air mixtures of 15–28% ammonia by volume in air.[40]When mixed withoxygen,it burns with a pale yellowish-green flame. Ignition occurs whenchlorineis passed into ammonia, forming nitrogen andhydrogen chloride;if chlorine is present in excess, then the highly explosivenitrogen trichloride(NCl3) is also formed.
Thecombustionof ammonia to form nitrogen and water isexothermic:
- 4 NH3+ 3 O2→ 2 N2+ 6 H2O(g),ΔH°r= −1267.20 kJ (or −316.8 kJ/mol if expressed per mol ofNH3)
Thestandard enthalpy change of combustion,ΔH°c,expressed permoleof ammonia and with condensation of the water formed, is −382.81 kJ/mol. Dinitrogen is the thermodynamic product ofcombustion:allnitrogen oxidesare unstable with respect toN2andO2,which is the principle behind thecatalytic converter.Nitrogen oxides can be formed askinetic productsin the presence of appropriatecatalysts,a reaction of great industrial importance in the production ofnitric acid:
- 4 NH3+ 5 O2→ 4 NO + 6 H2O
A subsequent reaction leads toNO2:
- 2 NO + O2→ 2 NO2
The combustion of ammonia in air is very difficult in the absence of acatalyst(such asplatinumgauze or warmchromium(III) oxide), due to the relatively lowheat of combustion,a lower laminar burning velocity, highauto-ignition temperature,highheat of vapourization,and a narrowflammability range.However, recent studies have shown that efficient and stable combustion of ammonia can be achieved using swirl combustors, thereby rekindling research interest in ammonia as a fuel for thermal power production.[41]The flammable range of ammonia in dry air is 15.15–27.35% and in 100% relative humidity air is 15.95–26.55%.[42][clarification needed]For studying thekineticsof ammonia combustion, knowledge of a detailed reliable reaction mechanism is required, but this has been challenging to obtain.[43]
Precursor to organonitrogen compounds
editAmmonia is a direct or indirect precursor to mostmanufactured nitrogen-containing compounds.It is the precursor to nitric acid, which is the source for most N-substituted aromatic compounds.
Aminescan be formed by the reaction of ammonia withalkyl halidesor, more commonly, withalcohols:
- CH3OH + NH3→ CH3NH2+ H2O
Its ring-opening reaction withethylene oxidegiveethanolamine,diethanolamine,andtriethanolamine.
Amidescan be prepared by the reaction of ammonia withcarboxylic acidand their derivatives. For example, ammonia reacts withformic acid(HCOOH) to yieldformamide(HCONH2) when heated.Acyl chloridesare the most reactive, but the ammonia must be present in at least a twofold excess to neutralise thehydrogen chlorideformed.Estersandanhydridesalso react with ammonia to form amides. Ammonium salts of carboxylic acids can bedehydratedto amides by heating to 150–200 °C as long as no thermally sensitive groups are present.
Other organonitrogen compounds includealprazolam,ethanolamine,ethyl carbamateandhexamethylenetetramine.
Precursor to inorganic nitrogenous compounds
editNitric acidis generated via theOstwald processbyoxidationof ammonia with air over aplatinumcatalyst at 700–850 °C (1,292–1,562 °F), ≈9 atm.Nitric oxideandnitrogen dioxideare intermediate in this conversion:[44]
- NH3+ 2 O2→ HNO3+ H2O
Nitric acid is used for the production offertilisers,explosives,and many organonitrogen compounds.
The hydrogen in ammonia is susceptible to replacement by a myriad substituents. Ammonia gas reacts with metallicsodiumto givesodamide,NaNH2.[38]
With chlorine,monochloramineis formed.
Pentavalent ammonia is known as λ5-amine,nitrogen pentahydridedecomposes spontaneously into trivalent ammonia (λ3-amine) and hydrogen gas at normal conditions. This substance was once investigated as a possible solidrocket fuelin 1966.[45]
Ammonia is also used to make the following compounds:
- Hydrazine,in theOlin Raschig processand theperoxide process
- Hydrogen cyanide,in theBMA processand theAndrussow process
- Hydroxylamineandammonium carbonate,in theRaschig process
- Urea,in theBosch–Meiser urea processand inWöhler synthesis
- ammonium perchlorate,ammonium nitrate,andammonium bicarbonate
Ammonia is aligandformingmetal ammine complexes.For historical reasons, ammonia is namedamminein the nomenclature ofcoordination compounds.One notable ammine complex iscisplatin(Pt(NH3)2Cl2,a widely used anticancer drug. Ammine complexes ofchromium(III) formed the basis ofAlfred Werner's revolutionary theory on the structure of coordination compounds. Werner noted only twoisomers(fac- andmer-) of the complex[CrCl3(NH3)3]could be formed, and concluded the ligands must be arranged around the metal ion at theverticesof anoctahedron.
Ammonia forms 1:1adductswith a variety ofLewis acidssuch asI2,phenol,andAl(CH3)3.Ammonia is ahard base(HSAB theory) and itsE & C parametersare EB= 2.31 and CB= 2.04. Its relative donor strength toward a series of acids, versus other Lewis bases, can be illustrated byC-B plots.
Detection and determination
editAmmonia in solution
editAmmonia and ammonium salts can be readily detected, in very minute traces, by the addition ofNessler's solution,which gives a distinct yellow colouration in the presence of the slightest trace of ammonia or ammonium salts. The amount of ammonia in ammonium salts can be estimated quantitatively by distillation of the salts withsodium(NaOH) orpotassium hydroxide(KOH), the ammonia evolved being absorbed in a known volume of standardsulfuric acidand the excess of acid then determinedvolumetrically;or the ammonia may be absorbed inhydrochloric acidand the ammonium chloride so formed precipitated asammonium hexachloroplatinate,[NH4]2[PtCl6].[46]
Gaseous ammonia
editSulfur sticksare burnt to detect small leaks in industrial ammonia refrigeration systems. Larger quantities can be detected by warming the salts with a caustic alkali or withquicklime,when the characteristic smell of ammonia will be at once apparent.[46]Ammonia is an irritant and irritation increases with concentration; thepermissible exposure limitis 25ppm,and lethal above 500 ppm by volume.[47]Higher concentrations are hardly detected by conventional detectors, the type of detector is chosen according to the sensitivity required (e.g. semiconductor, catalytic, electrochemical). Holographic sensors have been proposed for detecting concentrations up to 12.5% in volume.[48]
In a laboratorial setting, gaseous ammonia can be detected by using concentrated hydrochloric acid or gaseous hydrogen chloride. A dense white fume (which isammonium chloridevapor) arises from the reaction between ammonia and HCl(g).[49]
Ammoniacal nitrogen (NH3–N)
editAmmoniacal nitrogen(NH3–N) is a measure commonly used for testing the quantity ofammoniumions, derived naturally from ammonia, and returned to ammonia via organic processes, in water or waste liquids. It is a measure used mainly for quantifying values inwaste treatmentandwater purificationsystems, as well as a measure of the health of natural and man-made water reserves. It is measured in units of mg/L (milligramperlitre).
History
editThe ancient Greek historianHerodotusmentioned that there wereoutcropsof salt in an area of Libya that was inhabited by a people called the 'Ammonians' (now theSiwa oasisin northwestern Egypt, where salt lakes still exist).[50][51]The Greek geographerStraboalso mentioned the salt from this region. However, the ancient authorsDioscorides,Apicius,Arrian,Synesius,andAëtius of Amidadescribed this salt as forming clear crystals that could be used for cooking and that were essentiallyrock salt.[52]Hammoniacus salappears in the writings ofPliny,[53]although it is not known whether the term is equivalent to the more modern sal ammoniac (ammonium chloride).[21][54][55]
The fermentation ofurineby bacteria produces asolution of ammonia;hence fermented urine was used inClassical Antiquityto wash cloth and clothing, to remove hair from hides in preparation for tanning, to serve as amordantin dying cloth, and to remove rust from iron.[56]It was also used byancient dentiststo wash teeth.[57][58][59]
In the form of sal ammoniac (نشادر,nushadir), ammonia was important to theMuslim alchemists.It was mentioned in theBook of Stones,likely written in the 9th century and attributed toJābir ibn Hayyān.[60]It was also important to the Europeanalchemistsof the 13th century, being mentioned byAlbertus Magnus.[21]It was also used bydyersin theMiddle Agesin the form of fermentedurineto alter the colour of vegetable dyes. In the 15th century,Basilius Valentinusshowed that ammonia could be obtained by the action of alkalis on sal ammoniac.[61]At a later period, when sal ammoniac was obtained by distilling the hooves and horns of oxen and neutralizing the resulting carbonate withhydrochloric acid,the name 'spirit of hartshorn' was applied to ammonia.[21][62]
Gaseous ammonia was first isolated byJoseph Blackin 1756 by reactingsal ammoniac(ammonium chloride) withcalcined magnesia(magnesium oxide).[63][64]It was isolated again byPeter Woulfein 1767,[65][66]byCarl Wilhelm Scheelein 1770[67]and byJoseph Priestleyin 1773 and was termed by him 'alkaline air'.[21][68]Eleven years later in 1785,Claude Louis Bertholletascertained its composition.[69][21]
The production of ammonia from nitrogen in the air (and hydrogen) was invented byFritz Haberand Robert LeRossignol. The patent was sent in 1909 (USPTO Nr 1,202,995) and awarded in 1916. Later,Carl Boschdeveloped the industrial method for ammonia production (Haber–Bosch process). It was first used on an industrial scale inGermanyduringWorld War I,[70]following the allied blockade that cut off the supply ofnitratesfromChile.The ammonia was used to produce explosives to sustain war efforts.[71]The Nobel Prize in Chemistry 1918 was awarded to Fritz Haber "for the synthesis of ammonia from its elements".
Before the availability of natural gas, hydrogen as a precursor toammonia productionwas produced via theelectrolysisof water or using thechloralkali process.
With the advent of thesteelindustry in the 20th century, ammonia became a byproduct of the production ofcokingcoal.
Applications
editFertiliser
editIn the US as of 2019[update],approximately 88% of ammonia was used asfertiliserseither as its salts, solutions or anhydrously.[72]When applied to soil, it helps provide increased yields ofcropssuch asmaizeandwheat.[73]30% of agricultural nitrogen applied in the US is in the form ofanhydrousammonia, and worldwide, 110 million tonnes are applied each year.[74] Solutions of ammonia ranging from 16% to 25% are used in thefermentationindustry as a source of nitrogen for microorganisms and to adjust pH during fermentation.[75]
Refrigeration–R717
editBecause of ammonia's vapourization properties, it is a usefulrefrigerant.[70]It was commonly used before the popularisation ofchlorofluorocarbons(Freons). Anhydrous ammonia is widely used in industrial refrigeration applications and hockey rinks because of its highenergy efficiencyand low cost. It suffers from the disadvantage of toxicity, and requiring corrosion resistant components, which restricts its domestic and small-scale use. Along with its use in modernvapour-compression refrigerationit is used in a mixture along with hydrogen and water inabsorption refrigerators.TheKalina cycle,which is of growing importance to geothermal power plants, depends on the wide boiling range of the ammonia–water mixture.
Ammonia coolant is also used in the radiators aboard theInternational Space Stationin loops that are used to regulate the internal temperature and enable temperature-dependent experiments.[76][77]The ammonia is under sufficient pressure to remain liquid throughout the process. Single-phase ammonia cooling systems also serve the power electronics in each pair of solar arrays.
The potential importance of ammonia as a refrigerant has increased with the discovery that vented CFCs and HFCs are potent and stable greenhouse gases.[78]
Antimicrobial agent for food products
editAs early as in 1895, it was known that ammonia was 'stronglyantiseptic... it requires 1.4 grams per litre to preservebeef tea(broth).'[79]In one study, anhydrous ammonia destroyed 99.999% ofzoonotic bacteriain three types ofanimal feed,but notsilage.[80][81]Anhydrous ammonia is currently used commercially to reduce or eliminatemicrobialcontamination ofbeef.[82][83] Lean finely textured beef (popularly known as 'pink slime') in the beef industry is made from fattybeef trimmings(c. 50–70% fat) by removing the fat using heat andcentrifugation,then treating it with ammonia to killE. coli.The process was deemed effective and safe by theUS Department of Agriculturebased on a study that found that the treatment reducesE. colito undetectable levels.[84]There have been safety concerns about the process as well as consumer complaints about the taste and smell of ammonia-treated beef.[85]
Fuel
editAmmonia has been used as fuel, and is a proposed alternative to fossil fuels and hydrogen. Being liquid at ambient temperature under its own vapour pressure and having high volumetric and gravimetric energy density, ammonia is considered a suitable carrier for hydrogen,[86]and may be cheaper than direct transport of liquid hydrogen.[87]
Compared to hydrogen, ammonia is easier to store. Compared tohydrogen as a fuel,ammonia is much more energy efficient, and could be produced, stored and delivered at a much lower cost than hydrogen, which must be kept compressed or as a cryogenic liquid.[88][89]The rawenergy densityof liquid ammonia is 11.5 MJ/L,[88]which is about a third that ofdiesel.
Ammonia can be converted back to hydrogen to be used to power hydrogen fuel cells, or it may be used directly within high-temperaturesolid oxidedirect ammonia fuel cells to provide efficient power sources that do not emitgreenhouse gases.[90][91]Ammonia to hydrogen conversion can be achieved through thesodium amideprocess[92]or the catalytic decomposition of ammonia using solid catalysts.[93]
Ammonia engines or ammonia motors, using ammonia as aworking fluid,have been proposed and occasionally used.[94]The principle is similar to that used in afireless locomotive,but with ammonia as the working fluid, instead of steam or compressed air. Ammonia engines were used experimentally in the 19th century byGoldsworthy Gurneyin the UK and theSt. Charles Avenue Streetcarline inNew Orleansin the 1870s and 1880s,[95]and duringWorld War IIammonia was used to power buses inBelgium.[96]
Ammonia is sometimes proposed as a practical alternative tofossil fuelforinternal combustion engines.[96][97][98][99]However, ammonia cannot be easily used in existingOtto cycleengines because of its very narrowflammability range.Despite this, several tests have been run.[100][101][102]Its highoctane ratingof 120[103]and low flame temperature[104]allows the use of high compression ratios without a penalty of highNOxproduction. Since ammonia contains no carbon, its combustion cannot producecarbon dioxide,carbon monoxide,hydrocarbons,orsoot.
Ammonia production currently creates 1.8% of global CO2emissions. 'Green ammonia' is ammonia produced by usinggreen hydrogen(hydrogen produced by electrolysis with electricity fromrenewableenergy), whereas 'blue ammonia' is ammonia produced usingblue hydrogen(hydrogen produced by steam methane reforming (= SMR) where the carbon dioxide has been captured and stored (cfr. carbon capture and storage = CCS).[105]
Rocket engines have also been fueled by ammonia. TheReaction Motors XLR99rocket engine that powered theX-15hypersonic research aircraft used liquid ammonia. Although not as powerful as other fuels, it left nosootin the reusable rocket engine, and its density approximately matches the density of the oxidiser,liquid oxygen,which simplified the aircraft's design.
In 2020,Saudi Arabiashipped 40metric tonsof liquid 'blue ammonia' to Japan for use as a fuel.[106]It was produced as a by-product by petrochemical industries, and can be burned without giving offgreenhouse gases.Its energy density by volume is nearly double that of liquid hydrogen. If the process of creating it can be scaled up via purely renewable resources, producing green ammonia, it could make a major difference inavoiding climate change.[107]The companyACWA Powerand the city ofNeomhave announced the construction of a green hydrogen and ammonia plant in 2020.[108]
Green ammonia is considered as a potential fuel for future container ships. In 2020, the companiesDSMEandMAN Energy Solutionsannounced the construction of an ammonia-based ship, DSME plans to commercialize it by 2025.[109]The use of ammonia as a potential alternative fuel foraircraftjet enginesis also being explored.[110]
Japan intends to implement a plan to develop ammonia co-firing technology that can increase the use of ammonia in power generation, as part of efforts to assist domestic and other Asian utilities to accelerate their transition tocarbon neutrality.[111] In October 2021, the first International Conference on Fuel Ammonia (ICFA2021) was held.[112][113]
In June 2022,IHI Corporationsucceeded in reducing greenhouse gases by over 99% during combustion of liquid ammonia in a 2,000-kilowatt-class gas turbine achieving truly CO2-free power generation.[114] In July 2022,Quadnations of Japan, the U.S., Australia and India agreed to promote technological development for clean-burning hydrogen and ammonia as fuels at the security grouping's first energy meeting.[115]As of 2022[update],however, significant amounts ofNOxare produced.[116]Nitrous oxidemay also be a problem as it is a "greenhouse gas that is known to possess up to 300 times the Global Warming Potential (GWP) of carbon dioxide".[117]
TheIEAforecasts that ammonia will meet approximately 45% of shipping fuel demands by 2050.[118]
At high temperature and in the presence of a suitablecatalystammonia decomposes into its constituent elements.[119]Decomposition of ammonia is a slightly endothermic process requiring 23 kJ/mol (5.5kcal/mol) of ammonia, and yieldshydrogenandnitrogengas.
Other
editRemediation of gaseous emissions
editAmmonia is used to scrubSO2from the burning of fossil fuels, and the resulting product is converted toammonium sulfatefor use as fertiliser. Ammonia neutralises the nitrogen oxide (NOx) pollutants emitted by diesel engines. This technology, called SCR (selective catalytic reduction), relies on avanadia-based catalyst.[120]
Ammonia may be used to mitigate gaseous spills ofphosgene.[121]
Stimulant
editAmmonia, as the vapour released bysmelling salts,has found significant use as a respiratory stimulant. Ammonia is commonly used in the illegal manufacture ofmethamphetaminethrough aBirch reduction.[123]The Birch method of making methamphetamine is dangerous because the alkali metal and liquid ammonia are both extremely reactive, and the temperature of liquid ammonia makes it susceptible to explosive boiling when reactants are added.[124]
Textile
editLiquid ammonia is used for treatment of cotton materials, giving properties likemercerisation,using alkalis. In particular, it is used for prewashing of wool.[125]
Lifting gas
editAt standard temperature and pressure, ammonia is less dense than atmosphere and has approximately 45–48% of the lifting power of hydrogen orhelium.Ammonia has sometimes been used to fill balloons as alifting gas.Because of its relatively high boiling point (compared to helium and hydrogen), ammonia could potentially be refrigerated and liquefied aboard anairshipto reduce lift and add ballast (and returned to a gas to add lift and reduce ballast).[126]
Fuming
editAmmonia has been used to darken quartersawn white oak in Arts & Crafts and Mission-style furniture. Ammonia fumes react with the naturaltanninsin thewoodand cause it to change colour.[127]
Safety
editThe USOccupational Safety and Health Administration (OSHA)has set a 15-minute exposure limit for gaseous ammonia of 35 ppm by volume in the environmental air and an 8-hour exposure limit of 25 ppm by volume.[129]TheNational Institute for Occupational Safety and Health(NIOSH) recently reduced the IDLH (Immediately Dangerous to Health or Life, the level to which a healthy worker can be exposed for 30 minutes without suffering irreversible health effects) from 500 ppm to 300 ppm based on recent more conservative interpretations of original research in 1943. The 1 hour IDLH limit is still 500 ppm. Other organisations have varying exposure levels. US Navy Standards [U.S. Bureau of Ships 1962] maximum allowable concentrations (MACs): for continuous exposure (60 days) is 25 ppm; for exposure of 1 hour is 400 ppm.[130]
Ammonia vapour has a sharp, irritating, pungent odor that acts as a warning of potentially dangerous exposure. The average odor threshold is 5 ppm, well below any danger or damage. Exposure to very high concentrations of gaseous ammonia can result in lung damage and death.[129]Ammonia is regulated in the US as a non-flammable gas, but it meets the definition of a material that is toxic by inhalation and requires a hazardous safety permit when transported in quantities greater than 3,500 US gallons (13,000 L; 2,900 imp gal).[131]
Liquid ammonia is dangerous because it ishygroscopicand because it can causecaustic burns.SeeGas carrier § Health effects of specific cargoes carried on gas carriersfor more information.
Toxicity
editThe toxicity of ammonia solutions does not usually cause problems for humans and other mammals, as a specific mechanism exists to prevent its build-up in the bloodstream. Ammonia is converted tocarbamoyl phosphateby the enzymecarbamoyl phosphate synthetase,and then enters theurea cycleto be either incorporated intoamino acidsor excreted in the urine.[132]Fishandamphibianslack this mechanism, as they can usually eliminate ammonia from their bodies by direct excretion. Ammonia even at dilute concentrations is highly toxic to aquatic animals, and for this reason it isclassifiedas"dangerous for the environment".Atmospheric ammonia plays a key role in the formation offine particulate matter.[133]
Ammonia is a constituent oftobacco smoke.[134]
Coking wastewater
editAmmonia is present in coking wastewater streams, as a liquid by-product of the production ofcokefromcoal.[135]In some cases, the ammonia is discharged to themarine environmentwhere it acts as a pollutant. TheWhyalla SteelworksinSouth Australiais one example of a coke-producing facility that discharges ammonia into marine waters.[136]
Aquaculture
editAmmonia toxicity is believed to be a cause of otherwise unexplained losses infish hatcheries.Excess ammonia may accumulate and cause alteration of metabolism or increases in the body pH of the exposed organism. Tolerance varies among fish species.[137]At lower concentrations, around 0.05 mg/L, un-ionised ammonia is harmful to fish species and can result in poor growth and feed conversion rates, reduced fecundity and fertility and increase stress and susceptibility to bacterial infections and diseases.[138]Exposed to excess ammonia, fish may suffer loss of equilibrium, hyper-excitability, increased respiratory activity and oxygen uptake and increased heart rate.[137]At concentrations exceeding 2.0 mg/L, ammonia causes gill and tissue damage, extreme lethargy, convulsions, coma, and death.[137][139]Experiments have shown that the lethal concentration for a variety of fish species ranges from 0.2 to 2.0 mg/L.[139]
During winter, when reduced feeds are administered to aquaculture stock, ammonia levels can be higher. Lower ambient temperatures reduce the rate of algal photosynthesis so less ammonia is removed by any algae present. Within an aquaculture environment, especially at large scale, there is no fast-acting remedy to elevated ammonia levels. Prevention rather than correction is recommended to reduce harm to farmed fish[139]and in open water systems, the surrounding environment.
Storage information
editSimilar topropane,anhydrousammonia boils below room temperature when at atmospheric pressure. A storage vessel capable of 250psi(1.7MPa) is suitable to contain the liquid.[140]Ammonia is used in numerous different industrial applications requiring carbon or stainless steel storage vessels. Ammonia with at least 0.2% by weight water content is not corrosive to carbon steel.NH3carbon steelconstruction storage tanks with 0.2% by weight or more of water could last more than 50 years in service.[141]Experts warn that ammonium compounds not be allowed to come in contact withbases(unless in an intended and contained reaction), as dangerous quantities of ammonia gas could be released.
Laboratory
editThe hazards of ammonia solutions depend on the concentration: 'dilute' ammonia solutions are usually 5–10% by weight (< 5.62 mol/L); 'concentrated' solutions are usually prepared at >25% by weight. A 25% (by weight) solution has a density of 0.907 g/cm3,and a solution that has a lower density will be more concentrated. TheEuropean Union classificationof ammonia solutions is given in the table.
| Concentration by weight (w/w) |
Molarity | Concentration mass/volume (w/v) |
GHS pictograms | H-phrases |
|---|---|---|---|---|
| 5–10% | 2.87–5.62 mol/L | 48.9–95.7 g/L | H314 | |
| 10–25% | 5.62–13.29 mol/L | 95.7–226.3 g/L | H314,H335,H400 | |
| >25% | >13.29 mol/L | >226.3 g/L | H314,H335,H400,H411 |
The ammonia vapour from concentrated ammonia solutions is severely irritating to the eyes and therespiratory tract,and experts warn that these solutions only be handled in afume hood.Saturated ('0.880'–see§ Properties) solutions can develop a significant pressure inside a closed bottle in warm weather, and experts also warn that the bottle be opened with care. This is not usually a problem for 25% ('0.900') solutions.
Experts warn that ammonia solutions not be mixed withhalogens,as toxic and/or explosive products are formed. Experts also warn that prolonged contact of ammonia solutions withsilver,mercuryoriodidesalts can also lead to explosive products: such mixtures are often formed inqualitative inorganic analysis,and that it needs to be lightly acidified but not concentrated (<6% w/v) before disposal once the test is completed.
Laboratory use of anhydrous ammonia (gas or liquid)
editAnhydrous ammonia is classified as toxic (T) and dangerous for the environment (N). The gas is flammable (autoignition temperature:651 °C) and can form explosive mixtures with air (16–25%). Thepermissible exposure limit(PEL) in the United States is 50ppm(35 mg/m3), while theIDLHconcentration is estimated at 300 ppm. Repeated exposure to ammonia lowers the sensitivity to the smell of the gas: normally the odour is detectable at concentrations of less than 50 ppm, but desensitised individuals may not detect it even at concentrations of 100 ppm. Anhydrous ammonia corrodescopper- andzinc-containingalloys,which makesbrassfittings not appropriate for handling the gas. Liquid ammonia can also attackrubberand certain plastics.
Ammonia reacts violently with thehalogens.Nitrogen triiodide,aprimaryhigh explosive,is formed when ammonia comes in contact withiodine.Ammonia causes the explosivepolymerisationofethylene oxide.It also forms explosivefulminatingcompounds with compounds ofgold,silver,mercury,germaniumortellurium,and withstibine.Violent reactions have also been reported withacetaldehyde,hypochloritesolutions,potassium ferricyanideandperoxides.
Production
editGraphs are unavailable due to technical issues. There is more info onPhabricatorand onMediaWiki.org. |
Ammonia has one of the highest rates of production of any inorganic chemical. Production is sometimes expressed in terms of 'fixed nitrogen'. Global production was estimated as being 160 million tonnes in 2020 (147 tons of fixed nitrogen).[143]China accounted for 26.5% of that, followed by Russia at 11.0%, the United States at 9.5%, and India at 8.3%.[143]
Before the start ofWorld War I,most ammonia was obtained by thedry distillation[144]of nitrogenous vegetable and animal waste products, includingcameldung,where it wasdistilledby the reduction ofnitrous acidandnitriteswith hydrogen; in addition, it was produced by the distillation ofcoal,and also by the decomposition of ammonium salts byalkaline hydroxides[145]such asquicklime:[21]
For small scale laboratory synthesis, one can heatureaandcalcium hydroxideorsodium hydroxide:
- (NH2)2CO + Ca(OH)2→ CaCO3+ 2 NH3
Haber–Bosch
editTheHaber process,[146]also called the Haber–Bosch process, is the main industrial procedure for theproduction of ammonia.[147][148]It converts atmosphericnitrogen(N2) to ammonia (NH3) by a reaction withhydrogen(H2) using finely dividedironmetal as a catalyst:
This reaction is thermodynamically favorable at room temperature, but the kinetics are prohibitively slow. At high temperatures at which catalysts are active enough that the reaction proceeds to equilibrium, the reaction is reactant-favored rather than product-favored. As a result, high pressures are needed todrive the reaction forward.
The German chemistsFritz HaberandCarl Boschdeveloped the process in the first decade of the 20th century, and its improved efficiency over existing methods such as theBirkeland-EydeandFrank-Caroprocesses was a major advancement in the industrial production of ammonia.[149][150][151]The Haber process can be combined withsteam reformingto produce ammonia with just three chemical inputs: water, natural gas, and atmospheric nitrogen. Both Haber and Bosch were eventually awarded theNobel Prize in Chemistry:Haber in 1918 for ammonia synthesis specifically, and Bosch in 1931 for related contributions tohigh-pressure chemistry.Electrochemical
editAmmonia can be synthesized electrochemically. The only required inputs are sources of nitrogen (potentially atmospheric) and hydrogen (water), allowing generation at the point of use. The availability of renewable energy creates the possibility of zero emission production.[152][153]
'Greenammonia' is a name for ammonia produced from hydrogen that is in turn produced from carbon-free sources such as electrolysis of water. Ammonia from this source can be used as a liquid fuel with zero contribution to globalclimate change.
Anotherelectrochemicalsynthesis mode involves the reductive formation oflithium nitride,which can beprotonatedto ammonia, given aprotonsource, which can be hydrogen. In the early years of the development of this process,ethanolhas been used as such a source. The first use of this chemistry was reported in 1930, where lithium solutions in ethanol were used to produce ammonia at pressures of up to 1000 bar.[154]In 1994, Tsuneto et al. used lithium electrodeposition intetrahydrofuranto synthesize ammonia at more moderate pressures with reasonableFaradaic efficiency.[155]Subsequent studies have further explored the ethanol–tetrahydrofuran system for electrochemical ammonia synthesis.[156][157]Beyond simply mediating proton transfer to the nitrogen reduction reaction, ethanol has been found to play a multifaceted role, influencing electrolyte transformations and contributing to the formation of the solid electrolyte interphase, which enhances overall reaction efficiency[158][156]
In 2020, a solvent-agnosticgas diffusion electrodewas shown to improve nitrogen transport to the reactive lithium.NH3production rates of up to 30 ± 5 nmol/s/cm2and Faradaic efficiencies of up to 47.5 ± 4% at ambient temperature and 1 bar pressure were achieved.[159]
Ethanol can be replaced with a tetraalkylphosphonium salt.[160]The study observedNH3production rates of 53 ± 1 nmol/s/cm2at 69 ± 1% Faradaic efficiency experiments under 0.5-barhydrogen and 19.5-bar nitrogenpartial pressureat ambient temperature.[160]
In 2022, ammonia was produced via the lithium mediated process in a continuous-flow electrolyzer also demonstrating the hydrogen gas as proton source. The study synthesized ammonia at 61 ± 1% Faradaic efficiency at a current density of −6 mA/cm2at 1 bar and room temperature.[161]
Biochemistry and medicine
editAmmonia is essential for life.[163]For example, it is required for the formation ofamino acidsandnucleic acids,fundamental building blocks of life. Ammonia is however quite toxic. Nature thus uses carriers for ammonia. Within a cell,glutamateserves this role. In the bloodstream,glutamineis a source of ammonia.[164]
Ethanolamine, required for cell membranes, is the substrate forethanolamine ammonia-lyase,which produces ammonia:[165]
- H2NCH2CH2OH → NH3+ CH3CHO
Ammonia is both ametabolic wasteand a metabolic input throughout thebiosphere.It is an important source of nitrogen for living systems. Although atmospheric nitrogen abounds (more than 75%), few living creatures are capable of using atmospheric nitrogen in itsdiatomicform,N2gas. Therefore,nitrogen fixationis required for the synthesis of amino acids, which are the building blocks ofprotein.Some plants rely on ammonia and other nitrogenous wastes incorporated into the soil by decaying matter. Others, such as nitrogen-fi xinglegumes,benefit fromsymbioticrelationships withrhizobiabacteria that create ammonia from atmospheric nitrogen.[166]
In humans, inhaling ammonia in high concentrations can be fatal. Exposure to ammonia can causeheadaches,edema,impaired memory,seizuresandcomaas it isneurotoxicin nature.[167]
Biosynthesis
editIn certain organisms, ammonia is produced from atmospheric nitrogen byenzymescallednitrogenases.The overall process is callednitrogen fixation.Intense effort has been directed toward understanding the mechanism of biological nitrogen fixation. The scientific interest in this problem is motivated by the unusual structure of theactive siteof the enzyme, which consists of anFe7MoS9ensemble.[168]
Ammonia is also a metabolic product ofamino aciddeaminationcatalyzed by enzymes such asglutamate dehydrogenase 1.Ammonia excretion is common in aquatic animals. In humans, it is quickly converted tourea(byliver), which is much less toxic, particularly lessbasic.This urea is a major component of the dry weight ofurine.Most reptiles, birds, insects, and snails excreteuric acidsolely as nitrogenous waste.
Physiology
editAmmonia plays a role in both normal and abnormal animalphysiology.It isbiosynthesisedthrough normal amino acid metabolism and is toxic in high concentrations. Theliverconverts ammonia toureathrough a series of reactions known as theurea cycle.Liver dysfunction, such as that seen incirrhosis,may lead to elevated amounts of ammonia in the blood (hyperammonemia). Likewise, defects in the enzymes responsible for the urea cycle, such asornithine transcarbamylase,lead tohyperammonemia.Hyperammonemia contributes to the confusion andcomaofhepatic encephalopathy,as well as the neurological disease common in people with urea cycle defects andorganic acidurias.[169]
Ammonia is important for normal animal acid/base balance. After formation of ammonium fromglutamine,α-ketoglutaratemay be degraded to produce twobicarbonateions, which are then available as buffers for dietary acids. Ammonium is excreted in the urine, resulting in net acid loss. Ammonia may itself diffuse across therenal tubules,combine with a hydrogen ion, and thus allow for further acidexcretion.[170]
Excretion
editAmmonium ions are atoxicwaste product ofmetabolisminanimals.In fish and aquatic invertebrates, it is excreted directly into the water. In mammals, sharks, and amphibians, it is converted in theurea cycletourea,which is less toxic and can be stored more efficiently. In birds, reptiles, and terrestrial snails, metabolic ammonium is converted intouric acid,which is solid and can therefore be excreted with minimal water loss.[171]
Extraterrestrial occurrence
editAmmonia has been detected in the atmospheres of thegiant planetsJupiter,Saturn,UranusandNeptune,along with other gases such asmethane,hydrogen,andhelium.The interior ofSaturnmay include frozen ammonia crystals.[172]It is found onDeimosandPhobos–the twomoons of Mars.[citation needed]
Interstellar space
editAmmonia was first detected in interstellar space in 1968, based onmicrowaveemissions from the direction of thegalactic core.[173]This was the firstpolyatomicmolecule to be so detected. The sensitivity of the molecule to a broad range of excitations and the ease with which it can be observed in a number of regions has made ammonia one of the most important molecules for studies ofmolecular clouds.[174]The relative intensity of the ammonia lines can be used to measure the temperature of the emitting medium.
The following isotopic species of ammonia have been detected:NH3,15NH3,NH2D,NHD2,andND3.The detection of triplydeuteratedammonia was considered a surprise as deuterium is relatively scarce. It is thought that the low-temperature conditions allow this molecule to survive and accumulate.[175]
Since its interstellar discovery,NH3has proved to be an invaluable spectroscopic tool in the study of the interstellar medium. With a large number of transitions sensitive to a wide range of excitation conditions,NH3has been widely astronomically detected–its detection has been reported in hundreds of journal articles. Listed below is a sample of journal articles that highlights the range of detectors that have been used to identify ammonia.
The study of interstellar ammonia has been important to a number of areas of research in the last few decades. Some of these are delineated below and primarily involve using ammonia as an interstellar thermometer.
Interstellar formation mechanisms
editThe interstellar abundance for ammonia has been measured for a variety of environments. The [NH3]/[H2] ratio has been estimated to range from 10−7in small dark clouds[176]up to 10−5in the dense core of theOrion molecular cloud complex.[177]Although a total of 18 total production routes have been proposed,[178]the principal formation mechanism for interstellarNH3is the reaction:
- [NH4]++ e−→ NH3+ H
The rate constant,k,of this reaction depends on the temperature of the environment, with a value ofat 10 K.[179]The rate constant was calculated from the formula.For the primary formation reaction,a=1.05×10−6andB= −0.47.Assuming anNH+4abundance ofand an electron abundance of 10−7typical of molecular clouds, the formation will proceed at a rate of1.6×10−9cm−3s−1in a molecular cloud of total density105cm−3.[180]
All other proposed formation reactions have rate constants of between two and 13 orders of magnitude smaller, making their contribution to the abundance of ammonia relatively insignificant.[181]As an example of the minor contribution other formation reactions play, the reaction:
- H2+ NH2→ NH3+ H
has a rate constant of 2.2×10−15.AssumingH2densities of 105and [NH2]/[H2] ratio of 10−7,this reaction proceeds at a rate of 2.2×10−12,more than three orders of magnitude slower than the primary reaction above.
Some of the other possible formation reactions are:
- H−+ [NH4]+→ NH3+ H2
- [PNH3]++ e−→ P + NH3
Interstellar destruction mechanisms
editThere are 113 total proposed reactions leading to the destruction ofNH3.Of these, 39 were tabulated in extensive tables of the chemistry among C, N and O compounds.[182]A review of interstellar ammonia cites the following reactions as the principal dissociation mechanisms:[174]
| NH3+ [H3]+→ [NH4]++ H2 | (1) |
| NH3+ HCO+→ [NH4]++ CO | (2) |
with rate constants of 4.39×10−9[183]and 2.2×10−9,[184]respectively. The above equations (1,2) run at a rate of 8.8×10−9and 4.4×10−13,respectively. These calculations assumed the given rate constants and abundances of [NH3]/[H2] = 10−5,[[H3]+]/[H2] = 2×10−5,[HCO+]/[H2] = 2×10−9,and total densities ofn= 105,typical of cold, dense, molecular clouds.[185]Clearly, between these two primary reactions, equation (1) is the dominant destruction reaction, with a rate ≈10,000 times faster than equation (2). This is due to the relatively high abundance of[H3]+.
Single antenna detections
editRadio observations ofNH3from theEffelsberg 100-m Radio Telescopereveal that the ammonia line is separated into two components–a background ridge and an unresolved core. The background corresponds well with the locations previously detected CO.[186]The 25 mChilbolton telescopeinEnglanddetected radio signatures of ammonia inH II regions,HNH2Omasers,H–H objects, and other objects associated with star formation. A comparison of emission line widths indicates that turbulent or systematic velocities do not increase in the central cores of molecular clouds.[187]
Microwave radiation from ammonia was observed in several galactic objects including W3(OH),Orion A,W43,W51,and five sources in the galactic centre. The high detection rate indicates that this is a common molecule in the interstellar medium and that high-density regions are common in the galaxy.[188]
Interferometric studies
editVLAobservations ofNH3in seven regions with high-velocity gaseous outflows revealed condensations of less than 0.1pcin L1551, S140, andCepheus A.Three individual condensations were detected in Cepheus A, one of them with a highly elongated shape. They may play an important role in creating the bipolar outflow in the region.[189]
Extragalactic ammonia was imaged using the VLA inIC 342.The hot gas has temperatures above 70 K, which was inferred from ammonia line ratios and appears to be closely associated with the innermost portions of the nuclear bar seen in CO.[190]NH3was also monitored by VLA toward a sample of four galactic ultracompact HII regions: G9.62+0.19, G10.47+0.03, G29.96-0.02, and G31.41+0.31. Based upon temperature and density diagnostics, it is concluded that in general such clumps are probably the sites of massive star formation in an early evolutionary phase prior to the development of an ultracompact HII region.[191]
Infrared detections
editAbsorption at 2.97 micrometres due to solid ammonia was recorded from interstellar grains in theBecklin–Neugebauer Objectand probably in NGC 2264-IR as well. This detection helped explain the physical shape of previously poorly understood and related ice absorption lines.[192]
A spectrum of the disk of Jupiter was obtained from theKuiper Airborne Observatory,covering the 100 to 300 cm−1spectral range. Analysis of the spectrum provides information on global mean properties of ammonia gas and an ammonia ice haze.[193]
A total of 149 dark cloud positions were surveyed for evidence of 'dense cores' by using the (J,K) = (1,1) rotating inversion line of NH3.In general, the cores are not spherically shaped, with aspect ratios ranging from 1.1 to 4.4. It is also found that cores with stars have broader lines than cores without stars.[194]
Ammonia has been detected in theDraco Nebulaand in one or possibly two molecular clouds, which are associated with the high-latitude galacticinfrared cirrus.The finding is significant because they may represent the birthplaces for the Population I metallicity B-type stars in the galactic halo that could have been borne in the galactic disk.[195]
Observations of nearby dark clouds
editBy balancing and stimulated emission with spontaneous emission, it is possible to construct a relation betweenexcitation temperatureand density. Moreover, since the transitional levels of ammonia can be approximated by a 2-level system at low temperatures, this calculation is fairly simple. This premise can be applied to dark clouds, regions suspected of having extremely low temperatures and possible sites for future star formation. Detections of ammonia in dark clouds show very narrow lines – indicative not only of low temperatures, but also of a low level of inner-cloud turbulence. Line ratio calculations provide a measurement of cloud temperature that is independent of previous CO observations. The ammonia observations were consistent with CO measurements of rotation temperatures of ≈10 K. With this, densities can be determined, and have been calculated to range between 104and 105cm−3in dark clouds. Mapping ofNH3gives typical clouds sizes of 0.1pcand masses near 1 solar mass. These cold, dense cores are the sites of future star formation.
UC HII regions
editUltra-compact HII regions are among the best tracers of high-mass star formation. The dense material surrounding UCHII regions is likely primarily molecular. Since a complete study of massive star formation necessarily involves the cloud from which the star formed, ammonia is an invaluable tool in understanding this surrounding molecular material. Since this molecular material can be spatially resolved, it is possible to constrain the heating/ionising sources, temperatures, masses, and sizes of the regions. Doppler-shifted velocity components allow for the separation of distinct regions of molecular gas that can trace outflows and hot cores originating from forming stars.
Extragalactic detection
editAmmonia has been detected in external galaxies,[196][197]and by simultaneously measuring several lines, it is possible to directly measure the gas temperature in these galaxies. Line ratios imply that gas temperatures are warm (≈50 K), originating from dense clouds with sizes of tens of parsecs. This picture is consistent with the picture within ourMilky Waygalaxy – hot dense molecular cores form around newly forming stars embedded in larger clouds of molecular material on the scale of several hundred parsecs (giant molecular clouds; GMCs).
See also
edit- Ammonia (data page)– Chemical data page
- Ammonia fountain– Type of chemical demonstration
- Ammonia production– Overview of history and methods to produce NH3
- Ammonia solution– Chemical compound
- Cost of electricity by source– Comparison of costs of different electricity generation sources
- Forming gas– Mixture of hydrogen and nitrogen
- Haber process– Industrial process for ammonia production
- Hydrazine– Colorless flammable liquid with an ammonia-like odor
- Water purification– Process of removing impurities from water
References
edit- ^"Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005"(PDF).Archived(PDF)from the original on 9 October 2022.
- ^"Gases – Densities".The Engineering Toolbox.Retrieved3 March2016.
- ^Yost, Don M. (2007)."Ammonia and Liquid Ammonia Solutions".Systematic Inorganic Chemistry.Read Books. p. 132.ISBN978-1-4067-7302-6.
- ^Blum, Alexander (1975). "On crystalline character of transparent solid ammonia".Radiation Effects and Defects in Solids.24(4): 277.Bibcode:1975RadEf..24..277B.doi:10.1080/00337577508240819.
- ^"Ammonia".The American Chemical Society. 8 February 2021.Retrieved20 March2024.
- ^Perrin, D. D. (1982).Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution(2nd ed.). Oxford: Pergamon Press.
- ^Iwasaki, Hiroji; Takahashi, Mitsuo (1968). "Studies on the transport properties of fluids at high pressure".The Review of Physical Chemistry of Japan.38(1).
- ^abZumdahl, Steven S. (2009).Chemical Principles(6th ed.). Houghton Mifflin. p. A22.ISBN978-0-618-94690-7.
- ^"Ammonia, Anhydrous Safety Data Sheet"(PDF).University of Florida.Retrieved19 April2024.
- ^ab"Ammonia".Immediately Dangerous to Life or Health Concentrations (IDLH).National Institute for Occupational Safety and Health(NIOSH).
- ^Sigma-Aldrich Co.,Ammonia.
- ^NIOSH Pocket Guide to Chemical Hazards."#0028".National Institute for Occupational Safety and Health(NIOSH).
- ^Ritchie, Hannah."How many people does synthetic fertilizer feed?".Our World in Data.Retrieved4 September2021.
- ^"Ammonia Technology Roadmap – Analysis".IEA. 11 October 2021.
- ^"40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities".United States:Government Printing Office.
- ^"Global ammonia annual production capacity".Statistia.
- ^"Mitsubishi Heavy Industries BrandVoice: Scaling Ammonia Production for the World's Food Supply".Forbes.29 October 2021.
- ^Shreve, R. Norris;Brink, Joseph(1977).Chemical Process Industries(4th ed.). McGraw-Hill. p. 276.ISBN978-0-07-057145-7.
- ^"Pliny the Elder, The Natural History, BOOK XXXI. REMEDIES DERIVED FROM THE AQUATIC PRODUCTION, CHAP. 39. (7.)—THE VARIOUS KINDS OF SALT; THE METHODS OF PREPARING IT, AND THE REMEDIES DERIVED FROM IT. TWO HUNDRED AND FOUR OBSERVATIONS THERE UPON".perseus.tufts.edu.
- ^Hoover, Herbert (1950).Georgius Agricola De Re Metallica – Translated from the first Latin edition of 1556.New York: Dover Publications. p. 560.ISBN978-0486600062.
- ^abcdefghChisholm 1911,p. 861.
- ^Shannon, Francis Patrick (1938)Tables of the properties of aqua–ammonia solutions. Part 1 of The Thermodynamics of Absorption Refrigeration.Lehigh University studies. Science and technology series
- ^An ammonia–water slurry may swirl below Pluto's icy surface.Purdue University (9 November 2015)
- ^"ammoniacal (adj.)".Oxford English Dictionary.July 2023.doi:10.1093/OED/3565252514.
- ^Pimputkar, Siddha; Nakamura, Shuji (January 2016)."Decomposition of supercritical ammonia and modeling of supercritical ammonia–nitrogen–hydrogen solutions with applicability toward ammonothermal conditions".The Journal of Supercritical Fluids.107:17–30.doi:10.1016/j.supflu.2015.07.032.
- ^Hewat, A. W.; Riekel, C. (1979). "The crystal structure of deuteroammonia between 2 and 180 K by neutron powder profile refinement".Acta Crystallographica Section A.35(4): 569.Bibcode:1979AcCrA..35..569H.doi:10.1107/S0567739479001340.
- ^Billaud, Gerard; Demortier, Antoine (December 1975)."Dielectric constant of liquid ammonia from -35 to + 50.deg. and its influence on the association between solvated electrons and cation".The Journal of Physical Chemistry.79(26): 3053–3055.doi:10.1021/j100593a053.ISSN0022-3654.
- ^Ammoniain Linstrom, Peter J.; Mallard, William G. (eds.);NIST Chemistry WebBook,NIST Standard Reference Database Number 69,National Institute of Standards and Technology, Gaithersburg (MD)
- ^Jepsen, S. Dee; McGuire, Kent (27 November 2017)."Safe Handling of Anhydrous Ammonia".Ohio State University Extension.
- ^"Medical Management Guidelines for Ammonia".Agency for Toxic Substances and Disease Registry.12 January 2017.
- ^Hawkins, Nehemiah (1909).Hawkins' Mechanical Dictionary: A Cyclopedia of Words, Terms, Phrases and Data Used in the Mechanic Arts, Trades and Sciences.T. Audel. p. 15.
- ^abcNeufeld, R.; Michel, R.; Herbst-Irmer, R.; Schöne, R.; Stalke, D. (2016). "Introducing a Hydrogen-Bond Donor into a Weakly Nucleophilic Brønsted Base: Alkali Metal Hexamethyldisilazides (MHMDS, M = Li, Na, K, Rb and Cs) with Ammonia".Chem. Eur. J.22(35): 12340–12346.doi:10.1002/chem.201600833.PMID27457218.
- ^abcCombellas, C; Kanoufi, F; Thiébault, A (2001). "Solutions of solvated electrons in liquid ammonia".Journal of Electroanalytical Chemistry.499:144–151.doi:10.1016/S0022-0728(00)00504-0.
- ^Audrieth, Ludwig F.; Kleinberg, Jacob (1953).Non-aqueous solvents.New York: John Wiley & Sons. p. 45.LCCN52-12057.
- ^Edwin M. Kaiser (2001). "Calcium–Ammonia".Encyclopedia of Reagents for Organic Synthesis.doi:10.1002/047084289X.rc003.ISBN978-0471936237.
- ^abHaynes, William M., ed. (2013).CRC Handbook of Chemistry and Physics(94th ed.).CRC Press.pp. 9–26.ISBN9781466571143.
- ^Cleeton, C. E.; Williams, N. H. (1934). "Electromagnetic Waves of 1.1 cm (0 in). Wave-Length and the Absorption Spectrum of Ammonia".Physical Review.45(4): 234.Bibcode:1934PhRv...45..234C.doi:10.1103/PhysRev.45.234.
- ^abcChisholm 1911,p. 862.
- ^Baker, H. B. (1894)."Influence of moisture on chemical change".J. Chem. Soc.65:611–624.doi:10.1039/CT8946500611.
- ^"Ammonia".PubChem.
- ^Kobayashi, Hideaki; Hayakawa, Akihiro; Somarathne, K.D. Kunkuma A.; Okafor, Ekenechukwu C. (2019)."Science and technology of ammonia combustion".Proceedings of the Combustion Institute.37(1): 109–133.Bibcode:2019PComI..37..109K.doi:10.1016/j.proci.2018.09.029.
- ^Khan, A.S.; Kelley, R.D.; Chapman, K.S.; Fenton, D.L. (1995).Flammability limits of ammonia–air mixtures.U.S.: U.S. DOE Office of Scientific and Technical Information.OSTI215703.
- ^Shrestha, Krishna P.; Seidel, Lars; Zeuch, Thomas; Mauss, Fabian (7 July 2018)."Detailed kinetic mechanism for the oxidation of ammonia including the formation and reduction of nitrogen oxides"(PDF).Energy & Fuels.32(10): 10202–10217.doi:10.1021/acs.energyfuels.8b01056.ISSN0887-0624.S2CID103854263.Archived(PDF)from the original on 9 October 2022.
- ^Holleman, A. F.; Wiberg, E. (2001).Inorganic Chemistry.San Diego: Academic Press.ISBN978-0-12-352651-9.
- ^Sterrett, K. F.; Caron, A. P. (1966)."High pressure chemistry of hydrogenous fuels".Northrop Space Labs. Archived fromthe originalon 23 August 2011.Retrieved24 December2009.
- ^abChisholm 1911,p. 863.
- ^(OSHA) Source: Sax, N. Irving (1984)Dangerous Properties of Industrial Materials.6th Ed. Van Nostrand Reinhold.ISBN0-442-28304-0.
- ^Hurtado, J. L. Martinez; Lowe, C. R. (2014). "Ammonia-Sensitive Photonic Structures Fabricated in Nafion Membranes by Laser Ablation".ACS Applied Materials & Interfaces.6(11): 8903–8908.doi:10.1021/am5016588.ISSN1944-8244.PMID24803236.
- ^Holleman, A. F.; Wiberg, Egon; Wiberg, Nils; Eagleson, Mary; Brewer, William; Aylett, Bernhard J., eds. (2001).Holleman-Wiberg inorganic chemistry.San Diego, Calif. London: Academic.ISBN978-0-12-352651-9.
- ^Herodotus with George Rawlinson, trans.,The History of Herodotus(New York, New York: Tandy-Thomas Co., 1909), vol.2, Book 4, § 181,pp. 304–305.
- ^The land of the Ammonians is mentioned elsewhere in Herodotus'Historyand inPausanias'Description of Greece:
- Herodotus with George Rawlinson, trans.,The History of Herodotus(New York, New York: Tandy-Thomas Co., 1909), vol. 1, Book 2, § 42,p. 245,vol. 2, Book 3, § 25,p. 73,and vol. 2, Book 3, § 26,p. 74.
- Pausanias with W.H.S. Jones, trans.,Description of Greece(London, England: William Heinemann Ltd., 1979), vol. 2, Book 3, Ch. 18, § 3, pp. 109 and111and vol. 4, Book 9, Ch. 16, § 1,p. 239.
- ^Kopp, Hermann,Geschichte der Chemie[History of Chemistry] (Braunschweig, (Germany): Friedrich Vieweg und Sohn, 1845), Part 3,p. 237.[in German]
- ^Chisholm 1911cites PlinyNat. Hist.xxxi. 39. See: Pliny the Elder with John Bostock and H. T. Riley, ed.s,The Natural History(London, England: H. G. Bohn, 1857), vol. 5, Book 31, § 39,p. 502.
- ^"Sal-ammoniac".Webmineral.Retrieved7 July2009.
- ^Pliny also mentioned that when some samples of what was purported to benatron(Latin:nitrum,impure sodium carbonate) were treated with lime (calcium carbonate) and water, thenatronwould emit a pungent smell, which some authors have interpreted as signifying that thenatroneither was ammonium chloride or was contaminated with it. See:
- Pliny with W.H.S. Jones, trans.,Natural History(London, England: William Heinemann Ltd., 1963), vol. 8, Book 31, § 46, pp. 448–449.From pp. 448–449:"Adulteratur in Aegypto calce, deprehenditur gusto. Sincerum enim statim resolvitur, adulteratum calce pungit et asperum[oraspersum]reddit odorem vehementer. "(In Egypt it [i.e., natron] is adulterated with lime, which is detected by taste; for pure natron melts at once, but adulterated natron stings because of the lime, and emits a strong, bitter odour [or: when sprinkled [(aspersum) with water] emits a vehement odour])
- Kidd, John,Outlines of Mineralogy(Oxford, England: N. Bliss, 1809), vol. 2,p. 6.
- Moore, Nathaniel Fish,Ancient Mineralogy: Or, An Inquiry Respecting Mineral Substances Mentioned by the Ancients:... (New York, New York: G. & C. Carvill & Co., 1834),pp. 96–97.
- ^See:
- Forbes, R.J.,Studies in Ancient Technology,vol. 5, 2nd ed. (Leiden, Netherlands: E.J. Brill, 1966), pp.19,48,and65.
- Moeller, Walter O.,The Wool Trade of Ancient Pompeii(Leiden, Netherlands: E.J. Brill, 1976),p. 20.
- Faber, G.A. (pseudonym of: Goldschmidt, Günther) (May 1938) "Dyeing and tanning in classical antiquity,"Ciba Review,9:277–312. Available at:Elizabethan Costume
- Smith, William,A Dictionary of Greek and Roman Antiquities(London, England: John Murray, 1875), article: "Fullo" (i.e., fullers or launderers),pp. 551–553.
- Rousset, Henri (31 March 1917)"The laundries of the Ancients,"Scientific American Supplement,83(2152): 197.
- Bond, Sarah E.,Trade and Taboo: Disreputable Professions in the Roman Mediterranean(Ann Arbor, Michigan: University of Michigan Press, 2016),p. 112.
- Binz, Arthur (1936) "Altes und Neues über die technische Verwendung des Harnes" (Ancient and modern [information] about the technological use of urine),Zeitschrift für Angewandte Chemie,49(23): 355–360. [in German]
- Witty, Michael (December 2016) "Ancient Roman urine chemistry,"Acta Archaeologica,87(1): 179–191. Witty speculates that the Romans obtained ammonia in concentrated form by adding wood ash (impurepotassium carbonate) to urine that had been fermented for several hours.Struvite(magnesium ammonium phosphate) is thereby precipitated, and the yield of struvite can be increased by then treating the solution withbittern,a magnesium-rich solution that is a byproduct of making salt from sea water. Roasting struvite releases ammonia vapours.
- ^Lenkeit, Roberta Edwards (23 October 2018).High Heels and Bound Feet: And Other Essays on Everyday Anthropology, Second Edition.Waveland Press. p. 72.ISBN978-1-4786-3841-4.
- ^Perdigão, Jorge (3 August 2016).Tooth Whitening: An Evidence-Based Perspective.Springer. p. 170.ISBN978-3-319-38849-6.
- ^Bonitz, Michael; Lopez, Jose; Becker, Kurt; Thomsen, Hauke (9 April 2014).Complex Plasmas: Scientific Challenges and Technological Opportunities.Springer Science & Business Media. p. 465.ISBN978-3-319-05437-7.
- ^Haq, Syed Nomanul (1995).Names, Natures and Things: The Alchemist Jabir Ibn Hayyan and His Kitab Al-Ahjar (Book of Stones).Springer.ISBN978-0-7923-3254-1.
- ^Spiritus salis urinæ(spirit of the salt of urine, i.e., ammonium carbonate) had apparently been produced before Valentinus, although he presented a new, simpler method for preparing it in his book: Valentinus, Basilius,Vier Tractätlein Fr. Basilii Valentini... [Four essays of Brother Basil Valentine... ] (Frankfurt am Main, (Germany): Luca Jennis, 1625),"Supplementum oder Zugabe"(Supplement or appendix), pp. 80–81:"Der Weg zum Universal, damit die drei Stein zusammen kommen."(The path to the Universal, so that the three stones come together.).From p. 81:"Der Spiritus salis Urinæ nimbt langes wesen zubereiten / dieser proceß aber ist waß leichter unnd näher auß dem Salz von Armenia,... Nun nimb sauberen schönen Armenischen Salz armoniac ohn alles sublimiren / thue ihn in ein Kolben / giesse ein Oleum Tartari drauff / daß es wie ein Muß oder Brey werde / vermachs baldt / dafür thu auch ein grosen vorlag / so lege sich als baldt der Spiritus Salis Urinæ im Helm an Crystallisch..."(Spirit of the salt of urine [i.e., ammonium carbonate] requires a long method [i.e., procedure] to prepare; this [i.e., Valentine's] process [starting] from the salt from Armenia [i.e., ammonium chloride], however, is somewhat easier and shorter... Now take clean nice Armenian salt, without sublimating all [of it]; put it in a [distillation] flask; pour oil of tartar [i.e., potassium carbonate that has dissolved only in the water that it has absorbed from the air] on it, [so] that it [i.e., the mixture] becomes like a mush or paste; assemble it [i.e., the distilling apparatus (alembic)] quickly; for that [purpose] connect a large receiving flask; then soon spirit of the salt of urine deposits as crystals in the "helmet" [i.e., the outlet for the vapours, which is atop the distillation flask]...)
See also: Kopp, Hermann,Geschichte der Chemie[History of Chemistry] (Braunschweig, (Germany): Friedrich Vieweg und Sohn, 1845), Part 3,p. 243.[in German] - ^Maurice P. Crosland (2004).Historical Studies in the Language of Chemistry.Courier Dover Publications. p. 72.ISBN978-0-486-43802-3.
- ^Black, Joseph (1893) [1755].Experiments upon magnesia alba, quick-lime, and other alcaline substances.Edinburgh: W.F. Clay.
- ^Jacobson, Mark Z. (23 April 2012).Air Pollution and Global Warming: History, Science, and Solutions.Cambridge University Press.ISBN9781107691155.
- ^"Woulfe's bottle".Chemistry World.Retrieved1 July2017.
- ^Woulfe, Peter (1 January 1767)."Experiments on the Distillation of Acids, Volatile Alkalies, &c. Shewing How They May be Condensed without Loss, and How Thereby We May Avoid Disagreeable and Noxious Fumes: In a Letter from Mr. Peter Woulfe, F. R. S. to John Ellis, Esq; F. R. S."Philosophical Transactions.57:517–536.Bibcode:1767RSPT...57..517W.doi:10.1098/rstl.1767.0052.ISSN0261-0523.
- ^Urdang, George (1942).Pictorial life history of the apothecary chemist Carl Wilhelm Scheele.American Institute of the History of Pharmacy.hdl:1811/28946/Pictorial%20Life%20History_Scheele.pdf.
- ^See:
- Priestley, Joseph (1773)"Extrait d'une lettre de M. Priestley, en date du 14 Octobre 1773"(Extract of a letter from Mr. Priestley, dated 14 October 1773),Observations sur la Physique...,2:389.
- Priestley, Joseph,Experiments and Observations on Different Kinds of Air,vol. 1, 2nd ed. (London, England: 1775),Part 2, § 1: Observations on Alkaline Air, pp. 163–177.
- Schofield, Robert E.,The Enlightened Joseph Priestley: A Study of His Life and Work from 1773 to 1804(University Park, Pennsylvania: Pennsylvania State University Press, 2004),pp. 93–94.
- By 1775, Priestley had observed that electricity could decompose ammonia ( "alkaline air" ), yielding a flammable gas (hydrogen). See: Priestley, Joseph,Experiments and Observations on Different Kinds of Air,vol. 2 (London, England: J. Johnson, 1775),pp. 239–240.
- ^Berthollet (1785)"Analyse de l'alkali volatil"(Analysis of volatile alkali),Mémoires de l'Académie Royale des Sciences,316–326.
- ^abMax Appl (2006). "Ammonia".Ammonia, in Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a02_143.pub2.ISBN978-3527306732.
- ^Smith, Roland (2001).Conquering Chemistry.Sydney: McGraw-Hill.ISBN978-0-07-470146-1.
- ^"Mineral Commodity Summaries 2020, p. 117 – Nitrogen"(PDF).USGS.2020.Archived(PDF)from the original on 9 October 2022.Retrieved12 February2020.
- ^Lassaletta, Luis; Billen, Gilles; Grizzetti, Bruna; Anglade, Juliette; Garnier, Josette (2014)."50-year trends in nitrogen use efficiency of world cropping systems: the relationship between yield and nitrogen input to cropland".Environmental Research Letters.9(10): 105011.Bibcode:2014ERL.....9j5011L.doi:10.1088/1748-9326/9/10/105011.ISSN1748-9326.
- ^David Brown (18 April 2013)."Anhydrous ammonia fertilizer: abundant, important, hazardous".Washington Post.Retrieved23 April2013.
- ^"Applications of Anhydrous Ammonia and Aqueous Ammonia".mysoreammonia.Retrieved2 February2022.
- ^Wright, Jerry (13 April 2015)."Cooling System Keeps Space Station Safe, Productive".NASA.Archived fromthe originalon 12 January 2017.Retrieved1 July2017.
- ^"International Space Station's Cooling System: How It Works (Infographic)".Space.Retrieved1 July2017.
- ^"Reducing Hydrofluorocarbon (HFC) Use and Emissions in the Federal Sector through SNAP"(PDF).Archived(PDF)from the original on 9 October 2022.Retrieved2 December2018.
- ^Samuel Rideal (1895).Disinfection and Disinfectants: An Introduction to the Study of.London: Charles Griffin and Company. p.109.
- ^Tajkarimi, Mehrdad; Riemann, H. P.; Hajmeer, M. N.; Gomez, E. L.; Razavilar, V.; Cliver, D. O.; et al. (2008). "Ammonia disinfection of animal feeds – Laboratory study".International Journal of Food Microbiology.122(1–2): 23–28.doi:10.1016/j.ijfoodmicro.2007.11.040.PMID18155794.
- ^Kim, J. S.; Lee, Y. Y.; Kim, T. H. (January 2016). "A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass".Bioresource Technology.199:42–48.Bibcode:2016BiTec.199...42K.doi:10.1016/j.biortech.2015.08.085.PMID26341010.
- ^"Evaluation of Treatment Methods for Reducing Bacteria in Textured Beef",Jensen, Jean Let al.,American Society of Agricultural and Biological EngineersAnnual Meeting 2009
- ^Reference Document: Antimicrobial Interventions for Beef,Dawna Winkler and Kerri B. Harris, Center for Food Safety, Department of Animal Science,Texas A&M University,May 2009, page 12
- ^Moss, Michael (3 October 2009)."The Burger That Shattered Her Life".The New York Times.
- ^Moss, Michael (31 December 2009)."Safety of Beef Processing Method Is Questioned".The New York Times.
- ^"MOL studies ammonia FSRU concept".Offshore Energy.3 February 2022.Retrieved3 February2022.
- ^Collins (l_collins), Leigh (27 January 2022)."SPECIAL REPORT | Why shipping pure hydrogen around the world might already be dead in the water | Recharge".Recharge | Latest renewable energy news.Retrieved3 February2022.
- ^abLan, Rong; Tao, Shanwen (28 August 2014)."Ammonia as a suitable fuel for fuel cells".Frontiers in Energy Research.2:35.doi:10.3389/fenrg.2014.00035.
- ^Lindzon, Jared (27 February 2019)."He's Creating a New Fuel Out of Thin Air – for 85 Cents per Gallon".OZY.Archived fromthe originalon 26 April 2019.Retrieved26 April2019.
- ^Giddey, S.; Badwal, S. P. S.; Munnings, C.; Dolan, M. (10 October 2017). "Ammonia as a Renewable Energy Transportation Media".ACS Sustainable Chemistry & Engineering.5(11): 10231–10239.doi:10.1021/acssuschemeng.7b02219.
- ^Afif, Ahmed; Radenahmad, Nikdilila; Cheok, Quentin; Shams, Shahriar; Hyun Kim, Jung; Azad, Abul (12 February 2016)."Ammonia-fed fuel cells: a comprehensive review".Renewable and Sustainable Energy Reviews.60:822–835.Bibcode:2016RSERv..60..822A.doi:10.1016/j.rser.2016.01.120.Retrieved1 January2021.
- ^David, William I. F.; Makepeace, Joshua W.; Callear, Samantha K.; Hunter, Hazel M. A.; Taylor, James D.; Wood, Thomas J.; Jones, Martin O. (24 September 2014)."Hydrogen Production from Ammonia Using Sodium Amide".Journal of the American Chemical Society.136(38): 13082–13085.doi:10.1021/ja5042836.ISSN0002-7863.PMID24972299.
- ^Lucentini, Ilaria; García Colli, Germán; Luzi, Carlos D.; Serrano, Isabel; Martínez, Osvaldo M.; Llorca, Jordi (5 June 2021)."Catalytic ammonia decomposition over Ni–Ru supported on CeO2for hydrogen production: Effect of metal loading and kinetic analysis ".Applied Catalysis B: Environmental.286:119896.Bibcode:2021AppCB.28619896L.doi:10.1016/j.apcatb.2021.119896.hdl:2117/364129.ISSN0926-3373.S2CID233540470.
- ^Douglas Self(1 October 2007)."Ammonia Motors".Retrieved28 November2010.
- ^Louis C. Hennick; Elbridge Harper Charlton (1965).The Streetcars of New Orleans.Pelican Publishing. pp. 14–16.ISBN9781455612598.
- ^ab"Ammonia as a Transportation Fuel IV"(PDF).Norm Olson – Iowa Energy Center. 15–16 October 2007. Archived fromthe original(PDF)on 7 February 2012.
- ^Lee, Dongeun; Min, Hyungeun; Park, Hyunho; Song, Han Ho (1 November 2017)."Development of new combustion strategy for internal combustion engine fueled by pure ammonia"(PDF).Seoul National University, Department of Mechanical Engineering.Archived(PDF)from the original on 9 October 2022.Retrieved29 January2019.
- ^Brohi, Emtiaz Ali (2014)."Ammonia as fuel for internal combustion engines?"(PDF).Chalmers University of Technology.Archived(PDF)from the original on 9 October 2022.Retrieved29 January2019.
- ^Elucidare (2 February 2008)."Ammonia: New possibilities for hydrogen storage and transportation"(PDF).Elucidare Limited.Archived(PDF)from the original on 8 October 2010.
- ^Ammonia Powered CaronYouTube
- ^"Watch 'Ammonia Fuel'".Greg Vezina.Retrieved7 July2009.
- ^"Welcome to NH3 Car".NH3Car.
- ^"Ammonia".chm.bris.ac.uk.Retrieved3 March2016.
- ^Zacharakis-Jutz, George; Kong, Song-Charng (2013)."Characteristics of an SI Engine Using Direct Ammonia Injection"(PDF).Department of Mechanical Engineering, Iowa State University.Archived(PDF)from the original on 9 October 2022.Retrieved29 January2019.
- ^"Green ammonia | Royal Society".royalsociety.org.
- ^"Saudi Arabia Sends Blue Ammonia to Japan in World-First Shipment".Bloomberg.27 September 2020.Retrieved28 September2020.
- ^Service, Robert F. (12 July 2018)."Ammonia—a renewable fuel made from sun, air, and water—could power the globe without carbon".Science | AAAS.Retrieved28 September2020.
- ^"Will Saudi Arabia build the world's largest green hydrogen and ammonia plant?".energypost.eu.17 September 2020.Retrieved9 October2020.
- ^"DSME gets LR AIP for ammonia-fueled 23,000 TEU boxship".Offshore Energy.6 October 2020.Retrieved9 October2020.
- ^"What will power aircraft in the future?".Aviafuture.30 March 2022.Retrieved24 May2022.
- ^"Japan to advance ammonia co-firing technology".Argus Media.24 June 2021.Retrieved8 November2021.
- ^"First International Conference on Fuel Ammonia 2021".ICFA.6 October 2021. Archived fromthe originalon 7 November 2021.Retrieved7 November2021.
- ^"First International Conference on Fuel Ammonia Held".METI, Japan.12 October 2021.Retrieved7 November2021.
- ^"CO2-free power generation achieved with the world's first gas turbine using 100% liquid ammonia "(Press release).IHI Corporation.16 June 2022.Retrieved1 July2022.
- ^Masaya Kato (14 July 2022)."Quad members agree to promote hydrogen, ammonia fuel tech".The Nikkei.Retrieved14 July2022.
- ^"On the use of ammonia as a fuel – A perspective"(PDF).
- ^"Nitrogen Oxides as a By-product of Ammonia/Hydrogen Combustion Regimes"(PDF).
- ^Mehta, Amgeli (15 May 2023)."In the voyage to net-zero, which green shipping fuel will rule the seas?".Reuters.
- ^White, Alfred H.; Melville, Wm. (April 1905)."The Decomposition of Ammonia at High Temperatures".Journal of the American Chemical Society.27(4): 373–386.doi:10.1021/ja01982a005.ISSN0002-7863.
- ^"Diesel: Greener Than You Think".Archived fromthe originalon 10 May 2008.Retrieved7 July2009.
- ^"Phosgene: Health and Safety Guide".International Programme on Chemical Safety.1998.
- ^"Anhydrous ammonia tank locks have flaws".Cedar Rapids Gazette.6 October 2009.
- ^"Illinois Attorney General | Basic Understanding of Meth".Illinoisattorneygeneral.gov. Archived fromthe originalon 10 September 2010.Retrieved21 May2011.
- ^Greenberg, Michael I. (1 January 2003).Occupational, Industrial, and Environmental Toxicology.Elsevier Health Sciences.ISBN978-0323013406.
- ^Włochowicz, A.; Stelmasiak, E. (1983). "Change in thermal properties of wool after treatment with liquid ammonia".Journal of Thermal Analysis and Calorimetry.26(1): 17.doi:10.1007/BF01914084.S2CID96930751.
- ^Horkheimer, Donald (2005)."Ammonia – A Solution for Airships Demanding Rapid Changes in Net Buoyancy".AIAA 5th ATIO and 16th Lighter-Than-Air Sys Tech. And Balloon Systems Conferences.doi:10.2514/6.2005-7393.ISBN978-1-62410-067-3.Retrieved27 October2022.
- ^Fuming white oak.woodweb
- ^minerals year book, vol. 3
- ^ab"Toxic FAQ Sheet for Ammonia"(PDF).Agency for Toxic Substances and Disease Registry(ATSDR). September 2004.Archived(PDF)from the original on 9 October 2022.
- ^Ammonia,IDLH Documentation
- ^Is Anhydrous Ammonia covered under the Hazardous Materials Safety Permit Program?from the website of theUnited States Department of Transportation(DOT)
- ^Berg, J. M.; Tymoczko, J. L.; Stryer, L. (2002). "23.4: Ammonium Ion is Converted into Urea in Most Terrestrial Vertebrates".Biochemistry(5th ed.).
- ^Wang, Mingyi; Kong, Weimeng; Marten, Ruby; He, Xu-Cheng; Chen, Dexian; Pfeifer, Joschka; Heitto, Arto; Kontkanen, Jenni; Dada, Lubna; Kürten, Andreas; Yli-Juuti, Taina (13 May 2020)."Rapid growth of new atmospheric particles by nitric acid and ammonia condensation".Nature.581(7807): 184–189.Bibcode:2020Natur.581..184W.doi:10.1038/s41586-020-2270-4.ISSN1476-4687.PMC7334196.PMID32405020.
- ^Talhout, Reinskje; Schulz, Thomas; Florek, Ewa; Van Benthem, Jan; Wester, Piet; Opperhuizen, Antoon (2011)."Hazardous Compounds in Tobacco Smoke".International Journal of Environmental Research and Public Health.8(12): 613–628.doi:10.3390/ijerph8020613.ISSN1660-4601.PMC3084482.PMID21556207.
- ^"Cutting-Edge Solutions For Coking Wastewater Reuse To Meet The Standard of Circulation Cooling Systems".wateronline.Retrieved16 January2016.
- ^Vasudevan Rajaram; Subijoy Dutta; Krishna Parameswaran (30 June 2005).Sustainable Mining Practices: A Global Perspective.CRC Press. p. 113.ISBN978-1-4398-3423-7.
- ^abcOram, Brian."Ammonia in Groundwater, Runoff, and Streams".The Water Centre.Retrieved3 December2014.
- ^Hargreaves, J.A.; Tucker, C.S. (2004).Managing ammonia in fish ponds.Southern Regional Aquaculture Center.
- ^abcSergeant, Chris (5 February 2014)."The Management of Ammonia Levels in an Aquaculture Environment".Water/Wastewater.Retrieved3 December2014.
- ^Electronic Code of Federal Regulations:Archived4 November 2011 at theWayback Machine.Ecfr.gpoaccess.gov. Retrieved on 22 December 2011.
- ^"Ammonia Tanks – Carbon and Stainless Steel Construction".ammoniatanks.Retrieved28 June2021.
- ^"Nitrogen Statistics and Information U.S. Geological Survey".usgs.gov.Retrieved24 January2023.
- ^ab"Nitrogen (Fixed)--Ammonia (2022)"(PDF).U.S. National Minerals Information Center.Retrieved24 January2023.
- ^"Nobel Prize in Chemistry (1918) – Haber–Bosch process".Retrieved7 July2009.
- ^"Chemistry of the Group 2 Elements – Be, Mg, Ca, Sr, Ba, Ra".BBC.co.uk.Retrieved7 July2009.
- ^Habers process chemistry.India: Arihant publications. 2018. p. 264.ISBN978-93-131-6303-9.
- ^Appl, M. (1982). "The Haber–Bosch Process and the Development of Chemical Engineering".A Century of Chemical Engineering.New York: Plenum Press. pp. 29–54.ISBN978-0-306-40895-3.
- ^Appl, Max (2006). "Ammonia".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a02_143.pub2.ISBN978-3527306732.
- ^Smil, Vaclav (2004).Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production(1st ed.). Cambridge, MA: MIT.ISBN978-0-262-69313-4.
- ^Hager, Thomas (2008).The Alchemy of Air: A Jewish genius, a doomed tycoon, and the scientific discovery that fed the world but fueled the rise of Hitler(1st ed.). New York, New York: Harmony Books.ISBN978-0-307-35178-4.
- ^Sittig, Marshall (1979).Fertilizer Industry: Processes, Pollution Control, and Energy Conservation.Park Ridge, New Jersey: Noyes Data Corp.ISBN978-0-8155-0734-5.
- ^Lavars, Nick (30 November 2021)."Green ammonia electrolysis breakthrough could finally kill Haber–Bosch".New Atlas.Archivedfrom the original on 30 November 2021.Retrieved3 December2021.
- ^Blaine, Loz (19 November 2021)."FuelPositive promises green ammonia at 60% the cost of today's gray".New Atlas.Archivedfrom the original on 19 November 2021.Retrieved3 December2021.
- ^Fichter, Fr.; Girard, Pierre; Erlenmeyer, Hans (1 December 1930)."Elektrolytische Bindung von komprimiertem Stickstoff bei gewöhnlicher Temperatur".Helvetica Chimica Acta.13(6): 1228–1236.doi:10.1002/hlca.19300130604.
- ^Tsuneto, Akira; Kudo, Akihiko; Sakata, Tadayoshi (4 March 1994)."Lithium-mediated electrochemical reduction of high pressure N2 to NH3".Journal of Electroanalytical Chemistry.367(1): 183–188.doi:10.1016/0022-0728(93)03025-K.ISSN1572-6657.
- ^abLazouski, Nikifar; Schiffer, Zachary J.; Williams, Kindle; Manthiram, Karthish (17 April 2019)."Understanding Continuous Lithium-Mediated Electrochemical Nitrogen Reduction".Joule.3(4): 1127–1139.Bibcode:2019Joule...3.1127L.doi:10.1016/j.joule.2019.02.003.ISSN2542-4351.S2CID107985507.
- ^Andersen, Suzanne Z.; Čolić, Viktor; Yang, Sungeun; Schwalbe, Jay A.; Nielander, Adam C.; McEnaney, Joshua M.; Enemark-Rasmussen, Kasper; Baker, Jon G.; Singh, Aayush R.; Rohr, Brian A.; Statt, Michael J. (June 2019)."A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements".Nature.570(7762): 504–508.Bibcode:2019Natur.570..504A.doi:10.1038/s41586-019-1260-x.hdl:10044/1/72812.ISSN1476-4687.PMID31117118.S2CID162182383.
- ^Bjarke Valbæk Mygind, Jon; Pedersen, Jakob B.; Li, Katja; Deissler, Niklas H.; Saccoccio, Mattia; Fu, Xianbiao; Li, Shaofeng; Sažinas, Rokas; Andersen, Suzanne Z.; Enemark-Rasmussen, Kasper; Vesborg, Peter C. K.; Doganli-Kibsgaard, Jakob; Chorkendorff, Ib (22 November 2023)."Is Ethanol Essential for the Lithium-Mediated Nitrogen Reduction Reaction?".ChemSusChem.16(22): e202301011.Bibcode:2023ChSCh..16E1011B.doi:10.1002/cssc.202301011.ISSN1864-5631.PMID37681646.
- ^Lazouski, Nikifar; Chung, Minju; Williams, Kindle; Gala, Michal L.; Manthiram, Karthish (1 May 2020)."Non-aqueous gas diffusion electrodes for rapid ammonia synthesis from nitrogen and water-splitting-derived hydrogen".Nature Catalysis.3(5): 463–469.doi:10.1038/s41929-020-0455-8.ISSN2520-1158.S2CID218495730.
- ^abSuryanto, Bryan H. R.; Matuszek, Karolina; Choi, Jaecheol; Hodgetts, Rebecca Y.; Du, Hoang-Long; Bakker, Jacinta M.; Kang, Colin S. M.; Cherepanov, Pavel V.; Simonov, Alexandr N.; MacFarlane, Douglas R. (11 June 2021)."Nitrogen reduction to ammonia at high efficiency and rates based on a phosphonium proton shuttle".Science.372(6547): 1187–1191.Bibcode:2021Sci...372.1187S.doi:10.1126/science.abg2371.ISSN0036-8075.PMID34112690.S2CID235396282.
- ^Fu, Xianbiao; Pedersen, Jakob B.; Zhou, Yuanyuan; Saccoccio, Mattia; Li, Shaofeng; Sažinas, Rokas; Li, Katja; Andersen, Suzanne Z.; Xu, Aoni; Deissler, Niklas H.; Mygind, Jon Bjarke Valbæk; Wei, Chao; Kibsgaard, Jakob; Vesborg, Peter C. K.; Nørskov, Jens K.; Chorkendorff, Ib (16 February 2022)."Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation".Science.379(6633): 707–712.doi:10.1126/science.adf4403.PMID36795804.
- ^Roth, Karl S."eMedicine Specialties > Metabolic Diseases > Hyperammonemia".Retrieved7 July2009.
- ^Mohiuddin, S. S.; Khattar, D. (2023)."Biochemistry, Ammonia".StatPearls.Treasure Island.PMID31082083.
- ^Nelson, David L.; Cox, Michael M. (2005).Principles of Biochemistry(4th ed.). New York: W. H. Freeman. p. 632-633.ISBN0-7167-4339-6.
- ^Shibata, Naoki; Tamagaki, Hiroko; Hieda, Naoki; Akita, Keita; Komori, Hirofumi; Shomura, Yasuhito; Terawaki, Shin-Ichi; Mori, Koichi; Yasuoka, Noritake; Higuchi, Yoshiki; Toraya, Tetsuo (2010)."Crystal Structures of Ethanolamine Ammonia-lyase Complexed with Coenzyme B12 Analogs and Substrates".Journal of Biological Chemistry.285(34): 26484–26493.doi:10.1074/jbc.M110.125112.PMC2924083.PMID20519496.
- ^Adjei, M. B.; Quesenberry, K. H.; Chamblis, C. G. (June 2002)."Nitrogen Fixation and Inoculation of Forage Legumes".University of Florida IFAS Extension. Archived fromthe originalon 20 May 2007.
- ^Identifying the direct effects of ammonia on the brain – PubMed
- ^Igarashi, Robert Y.; Laryukhin, Mikhail; Dos Santos, Patricia C.; Lee, Hong-In; Dean, Dennis R.; Seefeldt, Lance C.; Hoffman, Brian M. (May 2005). "Trapping H- Bound to the Nitrogenase FeMo-Cofactor Active Site during H2 Evolution: Characterization by ENDOR Spectroscopy".Journal of the American Chemical Society.127(17): 6231–6241.doi:10.1021/ja043596p.PMID15853328.
- ^Zschocke, Johannes; Hoffman, Georg (2004).Vademecum Metabolism.Schattauer Verlag.ISBN978-3794523856.
- ^Rose, Burton; Helmut Rennke (1994).Renal Pathophysiology.Baltimore: Williams & Wilkins.ISBN978-0-683-07354-6.
- ^Campbell, Neil A.;Jane B. Reece(2002)."44".Biology(6th ed.). San Francisco: Pearson Education, Inc. pp.937–938.ISBN978-0-8053-6624-2.
- ^Edited by Kirk Munsell. Image page credit Lunar and Planetary Institute. NASA. "NASA's Solar Exploration: Multimedia: Gallery: Gas Giant InteriorsArchived20 February 2006 at theWayback Machine".Retrieved 26 April 2006.
- ^Cheung, A. C.; Rank, D. M.; Townes, C. H.; Thornton, D. D.; Welch, W. J. (1968). "Detection of NH3molecules in the interstellar medium by their microwave emission ".Phys. Rev. Lett.21(25): 1701.Bibcode:1968PhRvL..21.1701C.doi:10.1103/PhysRevLett.21.1701.
- ^abHo, P. T. P.; Townes, C. H. (1983). "Interstellar ammonia".Annu. Rev. Astron. Astrophys.21(1): 239–70.Bibcode:1983ARA&A..21..239H.doi:10.1146/annurev.aa.21.090183.001323.
- ^Millar, T. J. (2003). "Deuterium Fractionation in Interstellar Clouds".Space Science Reviews.106(1): 73–86.Bibcode:2003SSRv..106...73M.doi:10.1023/A:1024677318645.S2CID189793190.
- ^Ungerechts, H.; Walmsley, C. M.; Winnewisser, G. (1980)."Ammonia and cyanoacetylene observations of the high-density core of L-183 (L-134-N)".Astron. Astrophys.88:259.Bibcode:1980A&A....88..259U.
- ^Genzel, R.; Downes, D.; Ho, P. T. P. (1982). "NH3in Orion-KL – A new interpretation ".Astrophysical Journal.259:L103.Bibcode:1982ApJ...259L.103G.doi:10.1086/183856.
- ^"The UMIST data for Astrochemistry".Retrieved7 July2009.
- ^Vikor, L.; Al-Khalili, A.; Danared, H.; Djuric, N.; Dunn, G. H.; Larsson, M.; Le Padellec, A.; Rosen, S.; Af Ugglas, M. (1999)."Branching fractions of dissociative recombination of NH4+ and NH2+ molecular ions".Astronomy and Astrophysics.344:1027.Bibcode:1999A&A...344.1027V.
- ^van Dishoeck, E. F.; Black, J. H. (1986)."Comprehensive models of diffuse interstellar clouds – Physical conditions and molecular abundances"(PDF).Astrophys. J. Suppl. Ser.62:109–145.Bibcode:1986ApJS...62..109V.doi:10.1086/191135.hdl:1887/1980.
- ^"astrochemistry.net".astrochemistry.net.Retrieved21 May2011.
- ^Prasad, S. S.; Huntress, W. T. (1980)."A model for gas phase chemistry in interstellar clouds".The Astrophysical Journal Supplement Series.43:1.Bibcode:1980ApJS...43....1P.doi:10.1086/190665.
- ^Lininger, W.; Albritton, D. L.; Fehsenfeld, F. C.; Schmeltekopf, A. L.; Ferguson, E. E. (1975). "Flow–drift tube measurements of kinetic energy dependences of some exothermic proton transfer rate constants".J. Chem. Phys.62(9): 3549.Bibcode:1975JChPh..62.3549L.doi:10.1063/1.430946.
- ^Smith, D.; Adams, N. G. (1977). "Reactions of CH+nIONS with ammonia at 300 K ".Chemical Physics Letters.47(1): 145.Bibcode:1977CPL....47..145S.doi:10.1016/0009-2614(77)85326-8.
- ^Wooten, A.; Bozyan, E. P.; Garrett, D. B. (1980). "Detection of C2H in cold dark clouds ".Astrophysical Journal.239:844.Bibcode:1980ApJ...239..844W.doi:10.1086/158168.
- ^Wilson, T. L.; Downes, D.; Bieging, J. (1979). "Ammonia in Orion".Astronomy and Astrophysics.71(3): 275.Bibcode:1979A&A....71..275W.
- ^MacDonald, G. H.; Little, L. T.; Brown, A. T.; Riley, P. W.; Matheson, D. N.; Felli, M. (1981)."Detection of new ammonia sources".MNRAS.195(2): 387.Bibcode:1981MNRAS.195..387M.doi:10.1093/mnras/195.2.387.
- ^Morris, M.; Zuckerman, B.; Palmer, P.; Turner, B. E. (1973). "Interstellar ammonia".Astrophysical Journal.186:501.Bibcode:1973ApJ...186..501M.doi:10.1086/152515.
- ^Torrelles, J. M.; Ho, P. T. P.; Rodriguez, L. F.; Canto, J. (1985). "VLA observations of ammonia and continuum in regions with high-velocity gaseous outflows".Astrophysical Journal.288:595.Bibcode:1985ApJ...288..595T.doi:10.1086/162825.S2CID123014355.
- ^Ho, P. T. P.; Martin, R. N.; Turner, J. L.; Jackson, J. M. (1990)."VLA imaging of extragalactic ammonia – Hot gas in the nucleus of IC 342".Astrophysical Journal Letters.355:L19.Bibcode:1990ApJ...355L..19H.doi:10.1086/185728.
- ^Cesaroni, R.; Churchwell, E.; Hofner, P.; Walmsley, C. M.; Kurtz, S. (1994). "Hot ammonia toward compact HII regions".Astronomy and Astrophysics.288:903.Bibcode:1994A&A...288..903C.
- ^Knacke, R. F.; McCorkle, S.; Puetter, R. C.; Erickson, E. F.; Kraetschmer, W. (1982)."Observation of interstellar ammonia ice".Astrophysical Journal.260:141.Bibcode:1982ApJ...260..141K.doi:10.1086/160241.
- ^Orton, G. S.; Aumann, H. H.; Martonchik, J. V.; Appleby, J. F. (1982). "Airborne spectroscopy of Jupiter in the 100- to 300-cm−1region: Global properties of ammonia gas and ice haze ".Icarus.52(1): 81.Bibcode:1982Icar...52...81O.doi:10.1016/0019-1035(82)90170-1.
- ^Benson, P. J.; Myers, P. (1989)."A survey for dense cores in dark clouds".Astrophysical Journal Supplement Series.71:89.Bibcode:1989ApJS...71...89B.doi:10.1086/191365.
- ^Mebold, U.; Heithausen, A.; Reif, K. (1987). "Ammonia in the galactic halo and the infrared cirrus".Astronomy and Astrophysics.180:213.Bibcode:1987A&A...180..213M.
- ^Martin, R. N.; Ho, P. T. P. (1979). "Detection of extragalactic ammonia".Astronomy and Astrophysics.74(1): L7.Bibcode:1979A&A....74L...7M.
- ^Takano, S.; Nakai, N.; Kawaguchi, K. (1 April 2002)."Observations of Ammonia in External Galaxies I. NGC 253 and M 82".Publications of the Astronomical Society of Japan.54(2): 195–207.Bibcode:2002PASJ...54..195T.doi:10.1093/pasj/54.2.195.
Works cited
edit- "Aqua Ammonia".airgasspecialtyproducts. Archived fromthe originalon 19 November 2010.Retrieved28 November2010.
- This article incorporates text from a publication now in thepublic domain:Chisholm, Hugh,ed. (1911). "Ammonia".Encyclopædia Britannica.Vol. 1 (11th ed.). Cambridge University Press. pp. 861–863.
- Clark, Jim (April 2013) [2002]."The Haber Process".Retrieved15 December2018.
Further reading
edit- Bretherick, L., ed. (1986).Hazards in the Chemical Laboratory(4th ed.). London: Royal Society of Chemistry.ISBN978-0-85186-489-1.OCLC16985764.
- Greenwood, Norman N.;Earnshaw, Alan (1997).Chemistry of the Elements(2nd ed.).Butterworth-Heinemann.ISBN978-0-08-037941-8.
- Housecroft, C. E.; Sharpe, A. G. (2000).Inorganic Chemistry(1st ed.). New York: Prentice Hall.ISBN978-0-582-31080-3.
- Weast, R. C., ed. (1972).Handbook of Chemistry and Physics(53rd ed.). Cleveland, OH: Chemical Rubber Co.
External links
edit- International Chemical Safety Card 0414(anhydrous ammonia), ilo.org.
- International Chemical Safety Card 0215(aqueous solutions), ilo.org.
- CID 222fromPubChem
- "Ammoniac et solutions aqueuses"(in French). Institut National de Recherche et de Sécurité. Archived fromthe originalon 11 December 2010.
- Emergency Response to Ammonia Fertiliser Releases (Spills)for the Minnesota Department of Agriculture.ammoniaspills.org
- National Institute for Occupational Safety and Health–Ammonia Page,cdc.gov
- NIOSH Pocket Guide to Chemical Hazards–Ammonia,cdc.gov
- Ammonia, video


