Hematoxylin and eosin stain(orhaematoxylin and eosin stainorhematoxylin-eosin stain;often abbreviated asH&E stainorHE stain) is one of the principal tissuestainsused inhistology.[1][2][3]It is the most widely used stain inmedical diagnosis[1]and is often thegold standard.[4]For example, when apathologistlooks at abiopsyof a suspectedcancer,thehistological sectionis likely to be stained with H&E.

H&E is the combination of two histological stains:hematoxylinandeosin.The hematoxylin stains cellnucleia purplish blue, and eosin stains theextracellular matrixandcytoplasmpink, with other structures taking on different shades, hues, and combinations of these colors.[5][6]Hence a pathologist can easily differentiate between the nuclear and cytoplasmic parts of a cell, and additionally, the overall patterns of coloration from the stain show the general layout and distribution of cells and provides a general overview of a tissue sample's structure.[7]Thus, pattern recognition, bothby expert humans themselvesandby software that aids those experts(indigital pathology), provides histologic information.
This stain combination was introduced in 1877 by chemist Nicolaus Wissozky at theKazan Imperial Universityin Russia.[8][7]
Uses
editThe H&E staining procedure is the principal stain in histology[3][7][2][5]in part because it can be done quickly,[7]is not expensive, and stains tissues in such a way that a considerable amount ofmicroscopic anatomy[9][10]is revealed,[7][5][4]and can be used to diagnose a wide range ofhistopathologicconditions.[8]The results from H&E staining are not overly dependent on the chemical used tofixthe tissue or slight inconsistencies in laboratory protocol,[11]and these factors contribute to its routine use in histology.[7]
H&E staining does not always provide enough contrast to differentiate all tissues, cellular structures, or the distribution of chemical substances,[9]and in these cases more specific stains and methods are used.[10][7]
Method of application
editThere are many ways to prepare the hematoxylin solutions (formulation) used in the H&E procedure,[11][12][6]in addition, there are manylaboratory protocolsfor producing H&E stained slides,[9]some of which may be specific to a certain laboratory.[7]Although there is no standard procedure,[11][9]the results by convention are reasonably consistent in that cell nuclei are stained blue and thecytoplasmandextracellular matrixare stained pink.[7]Histology laboratories may also adjust the amount or type of staining for a particular pathologist.[7]
After tissues have been collected (often asbiopsies) and fixed, they are typically dehydrated and embedded in meltedparaffin wax,the resulting block is mounted on amicrotomeand cut into thin slices.[6]The slices are affixed to microscope slides at which point the wax is removed with a solvent and the tissue slices attached to the slides are rehydrated and are ready for staining.[6]Alternatively, H&E stain is the most used stain inMohs surgeryin which tissues are typically frozen, cut on acryostat(a microtome that cuts frozen tissue), fixed in alcohol, and then stained.[9]
The H&E staining method involves application ofhaematoxylinmixed with a metallic salt, ormordant,often followed by a rinse in a weak acid solution to remove excess staining (differentiation), followed bybluingin mildlyalkalinewater.[13][8][14]After the application of haematoxylin, the tissue iscounterstainedwith eosin (most commonlyeosin Y).[6][8][7]
Results
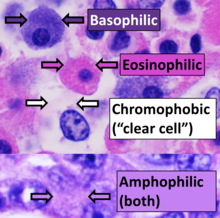
editHematoxylin principally colors thenucleiofcellsblue or dark-purple,[6][15][14]along with a few other tissues, such askeratohyalingranules andcalcifiedmaterial. Eosin stains thecytoplasmand some other structures includingextracellular matrixsuch ascollagen[5][7][14]in up to five shades of pink.[8]Theeosinophilic(substances that are stained by eosin)[5]structures are generally composed of intracellular or extracellularproteins.TheLewy bodiesandMallory bodiesare examples of eosinophilic structures. Most of thecytoplasmis eosinophilic and is rendered pink.[10][15]Red blood cellsare stained intensely red.[citation needed]
Mode of action
editAlthoughhematein,anoxidizedform of hematoxylin,[5][16][14]is the active colorant (when combined with a mordant), the stain is still referred to ashematoxylin.[8][13]Hematoxylin, when combined with a mordant (most commonly aluminumalum) is often considered to "resemble"[10]a basic, positively charged, orcationicstain.[5]Eosin is ananionic(negatively charged) and acidic stain.[5][10]The staining of nuclei byhemalum(a combination of aluminum ions and hematein)[14]is ordinarily due to binding of the dye-metal complex to DNA, but nuclear staining can be obtained after extraction of DNA[14]from tissue sections. The mechanism is different from that of nuclear staining by basic (cationic) dyes such asthionineortoluidine blue.[10]Staining by basic dyes occurs only from solutions that are less acidic than hemalum, and it is prevented by prior chemical or enzymatic extraction of nucleic acids. There is evidence to indicate that co-ordinate bonds, similar to those that hold aluminium and hematein together, bind the hemalum complex to DNA and to carboxy groups of proteins in the nuclearchromatin.[citation needed]
The structures do not have to be acidic or basic to be called basophilic and eosinophilic; the terminology is based on the affinity of cellular components for the dyes. Other colors, e.g. yellow and brown, can be present in the sample; they are caused by intrinsic pigments such asmelanin.Basal laminaeneed to be stained byPAS stainor somesilver stains,if they have to be well visible.Reticular fibersalso require silver stain. Hydrophobic structures also tend to remain clear; these are usually rich in fats, e.g.adipocytes,myelinaround neuronaxons,andGolgi apparatusmembranes.[citation needed]
Examples of H&E stained tissues
edit-
Bone,cell nuclei (blue-purple), bone matrix (pink).
-
Ductal carcinoma in situ(DCIS) in breast tissue, cell nuclei (blue-purple), extracellular material (pink).
-
Lung tissuetaken from anemphysemapatient. Cell nuclei (blue-purple), red blood cells (bright red), other cell bodies and extracellular material (pink), and air spaces (white).
-
Muscle tissue,cell nuclei (blue-purple), cell body (pink).
-
Basal cell carcinomaof theskin,cell nuclei (blue-purple), extracellular material (pink).
References
edit- ^abTitford, M. (2005). "The long history of hematoxylin".Biotechnic & Histochemistry.80(2): 73–80.doi:10.1080/10520290500138372.PMID16195172.S2CID20338201.
- ^abSmith C (2006)."Our debt to the logwood tree: the history of hematoxylin".MLO Med Lab Obs.38(5): 18, 20–2.PMID16761865.
- ^abDapson RW, Horobin RW (2009)."Dyes from a twenty-first century perspective".Biotech Histochem.84(4): 135–7.doi:10.1080/10520290902908802.PMID19384743.S2CID28563610.
- ^abRosai J (2007)."Why microscopy will remain a cornerstone of surgical pathology".Lab Invest.87(5): 403–8.doi:10.1038/labinvest.3700551.PMID17401434.
- ^abcdefghChan JK (2014)."The wonderful colors of the hematoxylin-eosin stain in diagnostic surgical pathology".Int J Surg Pathol.22(1): 12–32.doi:10.1177/1066896913517939.PMID24406626.S2CID26847314.
- ^abcdefStevens, Alan (1982). "The Haematoxylins". In Bancroft, John; Stevens, Alan (eds.).The Theory and Practice of Histological Techniques(2nd ed.). Longman Group Limited. p. 109.
- ^abcdefghijklWittekind D (2003)."Traditional staining for routine diagnostic pathology including the role of tannic acid. 1. Value and limitations of the hematoxylin-eosin stain".Biotech Histochem.78(5): 261–70.doi:10.1080/10520290310001633725.PMID14989644.S2CID10563849.
- ^abcdefTitford, Michael (2009)."Progress in the Development of Microscopical Techniques for Diagnostic Pathology".Journal of Histotechnology.32(1): 9–19.doi:10.1179/his.2009.32.1.9.ISSN0147-8885.S2CID26801839.
- ^abcdeLarson K, Ho HH, Anumolu PL, Chen TM (2011)."Hematoxylin and eosin tissue stain in Mohs micrographic surgery: a review".Dermatol Surg.37(8): 1089–99.doi:10.1111/j.1524-4725.2011.02051.x.PMID21635628.S2CID2538853.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^abcdefRoss, Michael H.; Pawlina, Wojciech (2016).Histology: a text and atlas: with correlated cell and molecular biology(7th ed.). Wolters Kluwer. pp. 984p.ISBN978-1451187427.
- ^abcSchulte EK (1991)."Standardization of biological dyes and stains: pitfalls and possibilities".Histochemistry.95(4): 319–28.doi:10.1007/BF00266958.PMID1708749.S2CID29628388.
- ^Llewellyn BD (2009)."Nuclear staining with alum hematoxylin".Biotech Histochem.84(4): 159–77.doi:10.1080/10520290903052899.PMID19579146.S2CID205713596.
- ^abOrtiz-Hidalgo C, Pina-Oviedo S (2019)."Hematoxylin: Mesoamerica's Gift to Histopathology. Palo de Campeche (Logwood Tree), Pirates' Most Desired Treasure, and Irreplaceable Tissue Stain".Int J Surg Pathol.27(1): 4–14.doi:10.1177/1066896918787652.PMID30001639.
- ^abcdefKiernan JA (2018)."Does progressive nuclear staining with hemalum (alum hematoxylin) involve DNA, and what is the nature of the dye-chromatin complex?".Biotech Histochem.93(2): 133–148.doi:10.1080/10520295.2017.1399466.PMID29320873.S2CID13481905.
- ^abLeeson, Thomas S.; Leeson, C. Roland (1981).Histology(Fourth ed.). W. B. Saunders Company. p. 600.ISBN978-0721657042.
- ^Kahr, Bart; Lovell, Scott; Subramony, Anand (1998). "The progress of logwood extract".Chirality.10(1–2): 66–77.doi:10.1002/chir.12.
Further reading
edit- Kiernan JA (2008) Histological and Histochemical Methods: Theory and Practice. 4th ed. Bloxham, UK: Scion.
- Lillie RD, Pizzolato P, Donaldson PT (1976) Nuclear stains with soluble metachrome mordant lake dyes. The effect of chemical endgroup blocking reactions and the artificial introduction of acid groups into tissues. Histochemistry 49: 23–35.
- Llewellyn BD (2009) Nuclear staining with alum-hematoxylin. Biotech. Histochem. 84: 159–177.
- Puchtler H, Meloan SN, Waldrop FS (1986) Application of current chemical concepts to metal-haematein and -brazilein stains. Histochemistry 85: 353–364.