Hydroxyzine,sold under the brand namesAtaraxandVistarilamong others, is anantihistaminemedication.[8]It is used in the treatment ofitchiness,anxiety,insomnia,andnausea(including that due tomotion sickness).[8]It is used eitherby mouthorinjection into a muscle.[8]
Hydroxyzine works by blocking the effects ofhistamine.[9]It is afirst-generation antihistaminein thepiperazinefamily of chemicals.[8][4]Common side effects includesleepiness,headache,anddry mouth.[8][9]Serious side effects may includeQT prolongation.[9]It is unclear if use during pregnancy or breastfeeding is safe.[8]
It was firstmadebyUnion Chimique Belgein 1956 and was approved for sale byPfizerin the United States later that year.[8][10]In 2022, it was the 46th most commonly prescribed medication in the United States, with more than 13million prescriptions.[11][12]
Medical uses
editHydroxyzine is used in the treatment ofitchiness,anxiety,andnauseadue tomotion sickness.[8]
Asystematic reviewconcluded that hydroxyzine outperformsplaceboin treating generalizedanxiety disorder.Insufficient data were available to compare the drug withbenzodiazepinesandbuspirone.[13]
Hydroxyzine can also be used for the treatment ofallergic conditions,such as chronicurticaria,atopicorcontact dermatoses,andhistamine-mediatedpruritus.[medical citation needed]These have also been confirmed in both recent and past studies to have no adverse effects on the liver, blood, nervous system, or urinary tract.[14][better source needed]
Use of hydroxyzine forpremedicationas a sedative has no effects ontropane alkaloids,such asatropine,but may, following general anesthesia, potentiatemeperidineandbarbiturates,and use in pre-anestheticadjunctive therapyshould be modified depending upon the state of the individual.[14]
Doses of hydroxyzine hydrochloride used for sleep range from 25 to 100 mg.[15][16][17]As with other antihistamine sleep aids, hydroxyzine is usually only prescribed for short term or "as-needed" use since tolerance to theCNS(central nervous system) effects of hydroxyzine can develop in as little as a few days.[18][non-primary source needed]A major systematic review andnetwork meta-analysisof medications for the treatment ofinsomniapublished in 2022 found little evidence to inform the use of hydroxyzine for insomnia.[19]A 2023 meta-review concludes that hydroxyzine is effective for inducing sleep onset but less effective for maintaining sleep for eight hours.[20]
Contraindications
editHydroxyzine is contraindicated forsubcutaneousorintra-articularadministration.[21]
The administration of hydroxyzine in large amounts by ingestion or intramuscular administration during the onset of pregnancy can causefetal abnormalities.When administered to pregnant rats, mice, and rabbits, hydroxyzine caused abnormalities such ashypogonadismwith doses significantly above that of the human therapeutic range.[22][better source needed]
In humans, a significant dose has not yet been established in studies, and, by default, theFood and Drug Administration(FDA) has introduced contraindication guidelines regarding hydroxyzine.[22]Use by those at risk for or showing previous signs ofhypersensitivityis also contraindicated.[22]
Other contraindications include the administration of hydroxyzine alongsidedepressantsand other compounds that affect thecentral nervous system;[22]if necessary, it should only be administered concomitantly in small doses.[22]If administered in small doses with other substances, as mentioned, then patients should refrain from using dangerous machinery, motor vehicles, or any other practice requiring absolute concentration, under safety laws.[22]
Studies have also been conducted which show that long-term prescription of hydroxyzine can lead totardive dyskinesiaafter years of use, but effects related todyskinesiahave also anecdotally been reported after periods of 7.5 months,[23]such as continual head rolling, lip licking, and other forms ofathetoidmovement. In certain cases, elderly patients' previous interactions withphenothiazinederivatives or pre-existingneuroleptictreatment may have contributed to dyskinesia at the administration of hydroxyzine due to hypersensitivity caused by prolonged treatment,[23]and therefore some contraindication is given for short-term administration of hydroxyzine to those with previous phenothiazine use.[23]
Side effects
editSeveral reactions have been noted in manufacturer guidelines—deep sleep, incoordination, sedation, calmness, and dizziness have been reported in children and adults, as well as others such ashypotension,tinnitus,and headaches.[24]Gastrointestinaleffects have also been observed, as well as less serious effects such as dryness of the mouth and constipation caused by the mildantimuscarinicproperties of hydroxyzine.[24]
Central nervous system effects such as hallucinations or confusion have been observed in rare cases, attributed mostly to overdosage.[25][24]Such properties have been attributed to hydroxyzine in several cases, particularly in patients treated for neuropsychological disorders, as well as in cases where overdoses have been observed. While there are reports of the "hallucinogenic" or "hypnotic" properties of hydroxyzine, several clinical data trials have not reported such side effects from the sole consumption of hydroxyzine, but rather, have described its overall calming effect described through the stimulation of areas within thereticular formation.The hallucinogenic or hypnotic properties have been described as being an additional effect from overall central nervous system suppression by other CNS agents, such aslithiumorethanol.[26]
Hydroxyzine exhibitsanxiolyticandsedativeproperties in many psychiatric patients. One study showed that patients reported very high levels of subjective sedation when first taking the drug, but that levels of reported sedation decreased markedly over 5–7 days, likely due to CNS receptor desensitization. Other studies have suggested that hydroxyzine acts as an acute hypnotic, reducingsleep onset latencyand increasing sleep duration — also showing that some drowsiness did occur. This was observed more in female patients, who also had greater hypnotic responses.[27]The use of sedating drugs alongside hydroxyzine can cause oversedation and confusion if administered at high doses—any form of hydroxyzine treatment alongside sedatives should be done under the supervision of a doctor.[28][25]
Because of the potential for more severe side effects, this drug is on the list to avoid in the elderly.[29]
Pharmacology
editPharmacodynamics
edit| Site | Ki(nM) | Species | Ref |
|---|---|---|---|
| 5-HT2A | 170 (IC50) | Rat | [31] |
| 5-HT2C | ND | ND | ND |
| α1 | 460 (IC50) | Rat | [31] |
| D1 | 10000+ | Mouse | [32] |
| D2 | 378 560 (IC50) |
Mouse Rat |
[32] [31] |
| H1 | 2.0–19 6.4 100 (IC50) |
Human Bovine Rat |
[33][34][35] [36] [31] |
| H2 | ND | ND | ND |
| H3 | ND | ND | ND |
| H4 | 10000+ | Human | [34] |
| mACh | 4600 10000+ 10000+ (IC50) 6310 (pA2) 3800 |
Human Mouse Rat Guinea pig Bovine |
[37] [32] [31] [38] [36] |
| VDCC | 3400+ (IC50) | Rat | [31] |
| Values are Ki(nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. | |||
Hydroxyzine's predominantmechanism of actionis as apotentandselectivehistamineH1receptorinverse agonist.[39][40]This action is responsible for itsantihistamineandsedativeeffects.[39][40]Unlike many other first-generation antihistamines, hydroxyzine has a loweraffinityfor themuscarinic acetylcholine receptors,and in accordance, has a lower risk ofanticholinergicside effects.[36][40][41][42]In addition to its antihistamine activity, hydroxyzine has also been shown to act more weakly as anantagonistof theserotonin5-HT2Areceptor,thedopamineD2receptor,and theα1-adrenergic receptor.[31][39]Similarly to theatypical antipsychotics,the comparably weakantiserotonergiceffects of hydroxyzine likely underlie its usefulness as ananxiolytic.[43]Other antihistamines without such properties have not been found to be effective in the treatment ofanxiety.[44]
Hydroxyzine crosses theblood–brain barriereasily and exerts effects in thecentral nervous system.[39]Apositron emission tomography(PET) study found that brain occupancy of the H1receptor was 67.6% for a single 30 mg dose of hydroxyzine.[45]In addition, subjective sleepiness correlated well with the brain H1receptor occupancy.[45]PET studies with antihistamines have found that brain H1receptor occupancy of more than 50% is associated with a high prevalence ofsomnolenceandcognitive decline,whereas brain H1receptor occupancy of less than 20% is considered to be non-sedative.[46]
Hydroxyzine also acts as a functional inhibitor ofacid sphingomyelinase.[47]
Pharmacokinetics
editHydroxyzine can be administered orally or via intramuscular injection. When given orally, hydroxyzine is rapidly absorbed from the gastrointestinal tract. Hydroxyzine is rapidly absorbed and distributed with oral and intramuscular administration, and is metabolized in the liver; the main metabolite (45%),cetirizine,is formed through oxidation of the alcohol moiety to a carboxylic acid byalcohol dehydrogenase,and overall effects are observed within one hour of administration. Higher concentrations are found in the skin than in the plasma. Cetirizine, although less sedating, is non-dialyzableand possesses similar antihistamine properties. The other metabolites identified include aN-dealkylated metabolite, and anO-dealkylated 1/16 metabolite with a plasma half-life of 59 hours. These pathways are mediated principally byCYP3A4andCYP3A5.[48][49]The N-dealykylated metabolite, norchlorcyclizine, bears some structural similarities totrazodone,but it has not been established whether it is pharmacologically active.[50][51]In animals, hydroxyzine and its metabolites are excreted in feces primarily through biliary elimination.[52][53]In rats, less than 2% of the drug is excreted unchanged.[53]
The time to reach maximum concentration (Tmax) of hydroxyzine is about 2.0 hours in both adults and children and itselimination half-lifeis around 20.0 hours in adults (mean age 29.3 years) and 7.1 hours in children.[5][6]Its elimination half-life is shorter in children compared to adults.[5]In another study, the elimination half-life of hydroxyzine in elderly adults was 29.3 hours.[7]One study found that the elimination half-life of hydroxyzine in adults was as short as 3 hours, but this may have just been due to methodological limitations.[54]Although hydroxyzine has a long elimination half-life and acts, in-vivo, as an antihistamine for as long as 24 hours, the predominant CNS effects of hydroxyzine and other antihistamines with long half-lives seem to diminish after 8 hours.[55]
Administration in geriatrics differs from the administration of hydroxyzine in younger patients; according to the FDA, there have not been significant studies made (2004), which include population groups over 65, which provide a distinction between elderly aged patients and other younger groups. Hydroxyzine should be administered carefully in the elderly with consideration given to possible reduced elimination.[25][better source needed]
Chemistry
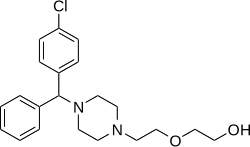
editHydroxyzine is a member of thediphenylmethylpiperazineclass of antihistamines.[medical citation needed]
Hydroxyzine is supplied mainly as adihydrochloridesalt(hydroxyzine hydrochloride) but also to a lesser extent as anembonatesalt (hydroxyzine pamoate).[56][57][58]Themolecular weightsof hydroxyzine, hydroxyzine dihydrochloride, and hydroxyzine pamoate are 374.9 g/mol, 447.8 g/mol, and 763.3 g/mol, respectively.[4]Due to their differences in molecular weight, 1 mg hydroxyzine dihydrochloride is equivalent to about 1.7 mg hydroxyzine pamoate.[59]
Analogues
editAnaloguesof hydroxyzine includebuclizine,cetirizine,cinnarizine,cyclizine,etodroxizine,meclizine,andpipoxizineamong others.
Society and culture
editBrand names
editHydroxyzinepreparationsrequire adoctor's prescription.The drug is available in twoformulations,thepamoateand thedihydrochlorideorhydrochloridesalts.Vistaril, Equipose, Masmoran, and Paxistil are preparations of the pamoate salt, while Atarax, Alamon, Aterax, Durrax, Tran-Q, Orgatrax, Quiess, and Tranquizine are of the hydrochloride salt.
See also
editReferences
edit- ^"Atarax: FDA-Approved Drugs".U.S.Food and Drug Administration(FDA).Retrieved25 March2023.
- ^"Vistaril: FDA-Approved Drugs".U.S.Food and Drug Administration(FDA).Retrieved5 August2020.
- ^Hubbard JR, Martin PR (2001).Substance Abuse in the Mentally and Physically Disabled.CRC Press. p. 26.ISBN9780824744977.
- ^abc"Hydroxyzine".United States National Library of Medicine(NLM).Retrieved4 March2020.
- ^abcdPaton DM, Webster DR (1985). "Clinical pharmacokinetics of H1-receptor antagonists (the antihistamines)".Clinical Pharmacokinetics.10(6):477–497.doi:10.2165/00003088-198510060-00002.PMID2866055.S2CID33541001.
- ^abSimons FE, Simons KJ, Frith EM (January 1984)."The pharmacokinetics and antihistaminic of the H1 receptor antagonist hydroxyzine".The Journal of Allergy and Clinical Immunology.73(1 Pt 1):69–75.doi:10.1016/0091-6749(84)90486-x.PMID6141198.
- ^abSimons KJ, Watson WT, Chen XY, Simons FE (January 1989). "Pharmacokinetic and pharmacodynamic studies of the H1-receptor antagonist hydroxyzine in the elderly".Clinical Pharmacology and Therapeutics.45(1):9–14.doi:10.1038/clpt.1989.2.PMID2562944.S2CID24571876.
- ^abcdefgh"Hydroxyzine Hydrochloride Monograph for Professionals".Drugs.American Society of Health-System Pharmacists.Retrieved21 November2018.
- ^abcBritish national formulary: BNF 74(74 ed.). British Medical Association. 2017. p. X.ISBN978-0857112989.
- ^Shorter E (2009).Before Prozac: the troubled history of mood disorders in psychiatry.Oxford [Oxfordshire]: Oxford University Press.ISBN9780195368741.
- ^"The Top 300 of 2022".ClinCalc.Archivedfrom the original on 30 August 2024.Retrieved30 August2024.
- ^"Hydroxyzine Drug Usage Statistics, United States, 2013 - 2022".ClinCalc.Retrieved30 August2024.
- ^Guaiana G, Barbui C, Cipriani A (December 2010). "Hydroxyzine for generalised anxiety disorder".The Cochrane Database of Systematic Reviews(12): CD006815.doi:10.1002/14651858.CD006815.pub2.PMID21154375.
- ^abUnited States Food & Drug Administration,(2004), p1
- ^Smith E, Narang P, Enja M, Lippmann S (2016)."Pharmacotherapy for Insomnia in Primary Care".The Primary Care Companion for CNS Disorders.18(2).doi:10.4088/PCC.16br01930.PMC4956432.PMID27486547.
- ^Matheson E, Hainer BL (July 2017). "Insomnia: Pharmacologic Therapy".American Family Physician.96(1):29–35.PMID28671376.
- ^Lippmann S, Yusufzie K, Nawbary MW, Voronovitch L, Matsenko O (2003). "Problems with sleep: what should the doctor do?".Comprehensive Therapy.29(1):18–27.doi:10.1007/s12019-003-0003-x.PMID12701339.S2CID1508856.
- ^Levander S, Ståhle-Bäckdahl M, Hägermark O (1 September 1991). "Peripheral antihistamine and central sedative effects of single and continuous oral doses of cetirizine and hydroxyzine".European Journal of Clinical Pharmacology.41(5):435–439.doi:10.1007/BF00626365.PMID1684750.S2CID25249362.
- ^De Crescenzo F, D'Alò GL, Ostinelli EG, Ciabattini M, Di Franco V, Watanabe N, et al. (July 2022)."Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis".Lancet.400(10347):170–184.doi:10.1016/S0140-6736(22)00878-9.hdl:11380/1288245.PMID35843245.S2CID250536370.
- ^Burgazli CR, Rana KB, Brown JN, Tillman F (March 2023)."Efficacy and safety of hydroxyzine for sleep in adults: Systematic review".Human Psychopharmacology.38(2): e2864.doi:10.1002/hup.2864.PMID36843057.
- ^"Hydroxyzine".
- ^abcdefUnited States Food & Drug Administration,(2004), p2
- ^abcClark BG, Araki M, Brown HW (April 1982). "Hydroxyzine-associated tardive dyskinesia".Annals of Neurology.11(4): 435.doi:10.1002/ana.410110423.PMID7103423.S2CID41117995.
- ^abcUCB South-Africa, et al., (2004)
- ^abcUnited States Food & Drug Administration,(2004), p3
- ^Marshik PL (2002)."Antihistamines".In Anderson PO, Knoben JE, Troutman WG (eds.).Handbook of Clinical Drug Data(10th ed.). New York:McGraw-HillMedical. pp. 794–796].ISBN978-0-07-136362-4.
- ^Alford C, Rombaut N, Jones J, Foley S, Idzikowski C, Hindmarch I (1992). "Acute effects of hydroxyzine on nocturnal sleep and sleep tendency the following day: A C-EEG study".Human Psychopharmacology.7(1):25–35.doi:10.1002/hup.470070104.S2CID143580519.
- ^Dolan CM (June 1958)."Management of emotional disturbances; use of hydroxyzine (atarax) in general practice".California Medicine.88(6):443–444.PMC1512309.PMID13536863.
- ^"NCQA's HEDIS Measure: Use of High Risk Medications in the Elderly"(PDF).NCQA.org.2008. Archived fromthe original(PDF)on 1 February 2010.Retrieved22 February2010.
- ^Roth BL,Driscol J."PDSP KiDatabase ".Psychoactive Drug Screening Program (PDSP).University of North Carolina at Chapel Hill and the United States National Institute of Mental Health.Retrieved14 August2017.
- ^abcdefgSnowman AM, Snyder SH (December 1990). "Cetirizine: actions on neurotransmitter receptors".The Journal of Allergy and Clinical Immunology.86(6 Pt 2):1025–1028.doi:10.1016/S0091-6749(05)80248-9.PMID1979798.
- ^abcHaraguchi K, Ito K, Kotaki H, Sawada Y, Iga T (June 1997)."Prediction of drug-induced catalepsy based on dopamine D1, D2, and muscarinic acetylcholine receptor occupancies".Drug Metabolism and Disposition.25(6):675–684.PMID9193868.
- ^Gillard M, Van Der Perren C, Moguilevsky N, Massingham R, Chatelain P (February 2002)."Binding characteristics of cetirizine and levocetirizine to human H(1) histamine receptors: contribution of Lys(191) and Thr(194)"(PDF).Molecular Pharmacology.61(2):391–399.doi:10.1124/mol.61.2.391.PMID11809864.S2CID13075815.Archived fromthe original(PDF)on 19 February 2019.
- ^abLim HD, van Rijn RM, Ling P, Bakker RA, Thurmond RL, Leurs R (September 2005). "Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist".The Journal of Pharmacology and Experimental Therapeutics.314(3):1310–1321.doi:10.1124/jpet.105.087965.PMID15947036.S2CID24248896.
- ^Anthes JC, Gilchrest H, Richard C, Eckel S, Hesk D, West RE, et al. (August 2002). "Biochemical characterization of desloratadine, a potent antagonist of the human histamine H(1) receptor".European Journal of Pharmacology.449(3):229–237.doi:10.1016/s0014-2999(02)02049-6.PMID12167464.
- ^abcKubo N, Shirakawa O, Kuno T, Tanaka C (March 1987)."Antimuscarinic effects of antihistamines: quantitative evaluation by receptor-binding assay".Japanese Journal of Pharmacology.43(3):277–282.doi:10.1254/jjp.43.277.PMID2884340.
- ^Cusack B, Nelson A, Richelson E (May 1994). "Binding of antidepressants to human brain receptors: focus on newer generation compounds".Psychopharmacology.114(4):559–565.doi:10.1007/bf02244985.PMID7855217.S2CID21236268.
- ^Orzechowski RF, Currie DS, Valancius CA (January 2005). "Comparative anticholinergic activities of 10 histamine H1 receptor antagonists in two functional models".European Journal of Pharmacology.506(3):257–264.doi:10.1016/j.ejphar.2004.11.006.PMID15627436.
- ^abcdSzepietowski J, Weisshaar E (2016). Itin P, Jemec GB (eds.).Itch - Management in Clinical Practice.Current Problems in Dermatology. Vol. 50. Karger Medical and Scientific Publishers. pp.1–80.ISBN9783318058895.
- ^abcHosák L, Hrdlička M (2017).Psychiatry and Pedopsychiatry.Charles University in Prague, Karolinum Press. p. 364.ISBN9788024633787.
- ^Berger FM (May 1957). "The chemistry and mode of action of tranquilizing drugs".Annals of the New York Academy of Sciences.67(10):685–700.Bibcode:1957NYASA..67..685B.doi:10.1111/j.1749-6632.1957.tb46006.x.PMID13459139.S2CID12702714.
- ^Tripathi KD (2013).Essentials of Medical Pharmacology.JP Medical Ltd. p. 165.ISBN9789350259375.
- ^Stein DJ, Hollander E, Rothbaum BO, eds. (2009).Textbook of Anxiety Disorders.American Psychiatric Publishing, Inc. p. 196.ISBN9781585622542.
- ^Lamberty Y, Gower AJ (September 2004). "Hydroxyzine prevents isolation-induced vocalization in guinea pig pups: comparison with chlorpheniramine and immepip".Pharmacology, Biochemistry, and Behavior.79(1):119–124.doi:10.1016/j.pbb.2004.06.015.PMID15388291.S2CID23593514.
- ^abTashiro M, Kato M, Miyake M, Watanuki S, Funaki Y, Ishikawa Y, et al. (October 2009). "Dose dependency of brain histamine H(1) receptor occupancy following oral administration of cetirizine hydrochloride measured using PET with [11C]doxepin".Human Psychopharmacology.24(7):540–548.doi:10.1002/hup.1051.PMID19697300.S2CID5596000.
- ^Yanai K, Tashiro M (January 2007). "The physiological and pathophysiological roles of neuronal histamine: an insight from human positron emission tomography studies".Pharmacology & Therapeutics.113(1):1–15.doi:10.1016/j.pharmthera.2006.06.008.PMID16890992.
- ^Sánchez-Rico M, Limosin F, Vernet R, Beeker N, Neuraz A, Blanco C, et al. (December 2021)."Hydroxyzine Use and Mortality in Patients Hospitalized for COVID-19: A Multicenter Observational Study".Journal of Clinical Medicine.10(24): 5891.doi:10.3390/jcm10245891.PMC8707307.PMID34945186.
- ^"Ucerax (hydroxyzine hydrochloride) 25 mg film-coated tablets. Summary of product characteristics"(PDF).Irish Medicines Board. 2013. Archived fromthe original(PDF)on 22 February 2014.Retrieved9 February2014.
- ^Foye WO, Lemke TL, Williams DA (2013).Foye's principles of medicinal chemistry(7th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins.ISBN978-1-60913-345-0.OCLC748675182.
- ^Thavundayil JX, Hambalek R, Kin NM, Krishnan B, Lal S (1994). "Prolonged penile erections induced by hydroxyzine: possible mechanism of action".Neuropsychobiology.30(1):4–6.doi:10.1159/000119126.PMID7969858.
- ^Malcolm MJ, Cody TE (January 1994). "Hydroxyzine and Possible Metabolites".Canadian Society of Forensic Science Journal.27(2):87–92.doi:10.1080/00085030.1994.10757029.
- ^"Vistaril (hydroxyzine pamoate) Capsules and Oral Suspension"(PDF).pfizer.2006. Archived fromthe original(PDF)on 3 July 2007.Retrieved7 March2007.
This paper says "The extent of renal excretion of Vistaril has not been determined"
- ^abPong SF, Huang CL (October 1974)."Comparative studies on distribution, excretion, and metabolism of hydroxyzine-3H and its methiodide-14C in rats".Journal of Pharmaceutical Sciences.63(10):1527–1532.doi:10.1002/jps.2600631008.PMID4436782.
- ^Kacew S (1989).Drug Toxicity & Metabolism In Pediatrics.CRC Press. p. 257.ISBN9780849345647.
- ^Simons FE (May 1994). "H1-receptor antagonists. Comparative tolerability and safety".Drug Safety.10(5):350–380.doi:10.2165/00002018-199410050-00002.PMID7913608.S2CID12749971.
- ^Elks J (14 November 2014).The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies.Springer. pp. 671–.ISBN978-1-4757-2085-3.
- ^Index Nominum 2000: International Drug Directory.Taylor & Francis. 2000. pp. 532–.ISBN978-3-88763-075-1.
- ^Morton IK, Hall JM (6 December 2012).Concise Dictionary of Pharmacological Agents: Properties and Synonyms.Springer Science & Business Media. pp. 147–.ISBN978-94-011-4439-1.
- ^"Hydroxyzine Pamoate 5 mg/ML Oral Suspension".Archived fromthe originalon 17 October 2020.