Irinotecan,sold under the brand nameCamptosaramong others, is ananti-cancer medicationused to treatcolon cancerandsmall cell lung cancer.[8]For colon cancer it is used either alone or withfluorouracil.[8]For small cell lung cancer it is used withcisplatin.[8]It is givenintravenously.[8]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Camptosar, Campto, Onivyde, others |
| AHFS/Drugs | Monograph |
| MedlinePlus | a608043 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokineticdata | |
| Bioavailability | NA |
| Metabolism | Liverglucuronidation |
| Eliminationhalf-life | 6 to 12 hours |
| Excretion | Bile ductandkidney |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.219.260 |
| Chemical and physical data | |
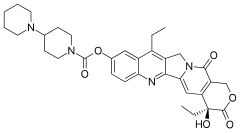
| Formula | C33H38N4O6 |
| Molar mass | 586.689g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Common side effects includediarrhea,vomiting,bone marrow suppression,hair loss, shortness of breath, and fever.[8]Other severe side effects includeblood clots,colon inflammation,andallergic reactions.[8]Those with two copies of theUGT1A1*28gene variant are at higher risk for side effects.[8]Use during pregnancy can result in harm to the baby.[8]Irinotecan is atopoisomerase inhibitor[9]—it blocks thetopoisomerase Ienzyme, resulting inDNA damageandcell death.[8]
Irinotecan was approved for medical use in the United States in 1996.[8]It is on theWorld Health Organization's List of Essential Medicines.[10]It is made from the natural compoundcamptothecinwhich is found in the Chinese ornamental treeCamptotheca acuminata.[8][11]
Medical uses
editIts main use is incolon cancer,in particular, in combination with other chemotherapy agents.[5]This includes the regimenFOLFIRI,which consists of infusional5-fluorouracil,leucovorin,and irinotecan. The regimen XELIRI consists ofcapecitabineand irinotecan.[12][13]
It may also be used together with fluorouracil and folinic acid forpancreatic cancerfollowing failure of initial treatment.[6]
In February 2024, the USFood and Drug Administration(FDA) approved irinotecan liposome, in combination withoxaliplatin,fluorouracil, and leucovorin, for the first-line treatment of metastatic pancreatic adenocarcinoma.[14][6]
Side effects
editThe most significant adverse effects of irinotecan include diarrhea, nausea and vomiting,neutropeniaand fever, infections of blood or lungs (sepsis, pneumonia), shock, dehydration, kidney failure and thrombocytopenia (low levels of blood platelets).[7][15]
Diarrhea
editEarly diarrhea occurs during or shortly after the infusion of Irinotecan, and is usually transient and infrequently severe. Late diarrhea occurs more than 24 hours after administration of Irinotecan and can be life threatening, sometimes leading to severe dehydration requiring hospitalization or intensive care unit admission. This side-effect is managed with the aggressive use of antidiarrheals such asloperamideoratropinewith the first loose bowel movement.[16][17]
Immunosuppression
editThe immune system is adversely impacted by irinotecan. This is reflected in loweredwhite blood cellcounts in the blood, in particular theneutrophils.[16][17]
Mechanism of action
editCamptothecin, one of the four major structural classifications of plant-derived anti-cancerous compounds, is a cytotoxic alkaloid which consists of a pentacyclic ring structure containing a pyrrole (3, 4 β) quinoline moiety, an S-configured lactone form, and a carboxylate form.[18]Camptothecin is an inhibitor of topoisomerase I. Its analogue, irinotecan, is activated by hydrolysis toSN-38,and is then inactivated byglucuronidationby uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1). The inhibition of topoisomerase I by the active metabolite SN-38 eventually leads to inhibition of both DNA replication and transcription.[15]
The molecular action of irinotecan occurs by trapping a subset oftopoisomerase-1-DNA cleavage complexes, those with a guanine +1 in the DNA sequence.[19]One irinotecan molecule stacks against the base pairs flanking the topoisomerase-induced cleavage site and poisons (inactivates) thetopoisomerase1 enzyme.[19]
Interactive pathway
editClick on genes, proteins and metabolites below to link to respective articles.[§ 1]
- ^The interactive pathway map can be edited at WikiPathways:"IrinotecanPathway_WP229".
Pharmacokinetics
editAdministration
editIrinotecan can be administered by 30- or 90-minute intravenous infusions of either 125 mg/m2weekly for four of every six weeks or 350 mg/m2every three weeks.[20]
Distribution
editIrinotecan is a hydrophilic compound with a large volume of distribution (400 L/m2).[20][21]At physiological pH, irinotecan and its active metabolite ethyl-10-hydroxy-camptothecin (SN-38) are present in two pH-dependent equilibrium isoforms; the anti tumor active lactone ring which hydrolyzed to the carboxylate isoform.[21]
In plasma, the majority of irinotecan and SN-38 are bound to albumin, which stabilizes their lactone forms. In blood, irinotecan and SN-38 are bound to platelets and red blood cells.[21]
Irinotecan has a linear pharmacokinetic. Population pharmacokinetic models assumed a three-compartmental model for irinotecan and a two-compartmental model for SN-38.[21]
SN-38 has a short distribution half-life (approximately 8 min). It reached its peak plasma concentration within 2 h after infusion. Also SN-38 exhibit a second peak in the plasma concentration because of its enterohepatic re-circulation and its release from erythrocytes.[21]
Metabolism
editActivation by carboxylesterases and butyrylcholinesteras
editAbout 2–5% of the pro-drug irinotecan is hydrolyzed into its active metabolite SN-38 in the liver by two carboxylesterase converting enzymes (CES1 and CES2) and in plasma by butyrylcholinesterase (hBChE).[21][22]CES2 has a 12.5-fold higher affinity for irinotecan than CES1. While, butyrylcholinesterase has a 6-fold higher activity for irinotecan than CES.[21]After conversion, SN-38 is actively transported to the liver by the organic anion transporting polypeptide (OATP) 1B1 transporter.[21][22]
Inactivation by uridine diphosphate glucuronosyltransferases
editSN-38 is inactivated by glucuronidation to SN-38G (β-glucuronide conjugate) by several uridine diphosphate glucuronosyltransferase enzymes (UGTs) in the liver (UGT1A1, UGT1A9) and extra-hepatic (UGT1A1, UGT1A7, UGT1A10) and excreted into the bile.[21][22]Several UGT polymorphisms affects irinotecan pharmacokinetics, for example, the decreased UGT1 activity, may lead to severe toxicity. Also, UGT1A1 conjugates bilirubin and bilirubin glucuronidation is another risk factor for increased toxicity[21]
De-conjugation by β-glucuronidases
editThe intestinal bacteria produced β-glucuronidases that de-conjugate SN-38G to SN-38 resulting in entero-hepatic re-circulation of SN-38.[21][22]
Metabolism by cytochrome P450 enzymes
editIrinotecan is metabolized by intrahepatic cytochrome P450 enzymes, CYP3A4 and CYP3A5 into inactive metabolites APC (7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino] carbonyloxycamptothecin) and NPC (7-ethyl-10-[4-amino-1-piperidino] carbonyloxycamptothecin). NPC can be further converted by CES1 and CES2 in the liver to SN-38.[21][22]Induction or inhibition of CYP3A enzymes by smoking, some herbs and medications may result in interactions with irinotecan.[21]
Transport to bile
editIrinotecan is transported to bile by the ATP-binding cassette (ABC) transporter proteins: ABCB1, ABCC1, ABCC2, and ABCG2.[21][22]
Elimination
editIrinotecan clearance is mainly biliary (66%) and estimated 12–21 L/h/m2.[21]All metabolites, except SN-38G, are mainly excreted in feces.[21][22]Irinotecan elimination half-lives were reported between 5 and 18 h. SN-38 half-lives were reported between 6 and 32 h.[21]
There is high (30%) interindividual variability in irinotecan pharmacokinetic parameters which can be altered by several factors including age, sex, dose, administration timing, hepatic function, enzyme activity or hematocrit levels.[21][22]
Pharmacogenomics
editIrinotecan is converted by an enzyme into its active metabolite SN-38, which is in turn inactivated by the enzyme UGT1A1 by glucuronidation.
*28 variant patients
editPeople with variants of the UGT1A1 called TA7,also known as the "*28 variant", express fewer UGT1A1 enzymes in their liver and often haveGilbert's syndrome.During chemotherapy, they effectively receive a larger than expected dose because their bodies are not able to clear irinotecan as fast as others. In studies this corresponds to higher incidences of severe neutropenia and diarrhea.[23]
In 2004, a clinical study was performed that both validated prospectively the association of the *28 variant with greater toxicity and the ability ofgenetic testingin predicting that toxicity before chemotherapy administration.[23]
In 2005, the FDA made changes to the labeling of irinotecan to addpharmacogenomicsrecommendations, such that irinotecan recipients with ahomozygous(both of the two gene copies) polymorphism in UGT1A1 gene, to be specific, the *28 variant, should be considered for reduced drug doses.[5]Irinotecan is one of the first widely used chemotherapy agents that is dosed according to the recipient's genotype.[24]
History
editIn February 2024, the FDA approved irinotecan liposome, in combination withoxaliplatin,fluorouracil, and leucovorin, for the first-line treatment of metastatic pancreatic adenocarcinoma.[14]Efficacy was evaluated in NAPOLI 3 (NCT04083235), a randomized, multicenter, open-label, active-controlled trial in 770 participants with metastatic pancreatic adenocarcinoma who had not previously received chemotherapy in the metastatic setting.[14]Randomization was stratified by region, liver metastases, and ECOG performance status.[14]Participants were randomized (1:1) to receive one of the following treatments: NALIRIFOX: irinotecan liposome 50 mg/m2 as an intravenous infusion over 90 minutes, followed by oxaliplatin 60 mg/m2 as an intravenous infusion over 120 minutes, followed by leucovorin 400 mg/m2 intravenously over 30 minutes, followed by fluorouracil 2400 mg/m2 intravenously over 46 hours, every 2 weeks; Gem+NabP: Nab-paclitaxel 125 mg/m2 as an intravenous infusion over 35 minutes, followed by gemcitabine 1000 mg/m2 intravenously over 30 minutes on days 1, 8, and 15 of each 28-day cycle.[14]The application was grantedorphan drugdesignation.[14]
Society and culture
editLegal status
editIrinotecan received accelerated approval from the USFood and Drug Administration(FDA) in 1996,[25]and full approval in 1998.[26][27][28]
A liposome encapsulated version of irinotecan sold under the brand name Onivyde, was approved by the FDA in October 2015, to treat metastaticpancreatic cancer.[29][30]It was approved for medical use in the European Union in October 2016.[7]
Names
editDuring development, it was known as CPT-11.[medical citation needed]
References
edit- ^"Prescription medicines: registration of new chemical entities in Australia, 2016".Therapeutic Goods Administration (TGA).21 June 2022.Retrieved10 April2023.
- ^"Product monograph brand safety updates".Health Canada.7 July 2016.Retrieved3 April2024.
- ^"Cancer therapies".Health Canada.8 May 2018.Retrieved13 April2024.
- ^"Onivyde pegylated liposomal 4.3 mg/ml concentrate for solution for infusion – Summary of Product Characteristics (SmPC)".(emc).18 February 2020.Retrieved25 May2020.
- ^abc"Camptosar- irinotecan hydrochloride injection, solution".DailyMed.10 February 2020.Retrieved25 May2020.
- ^abc"Onivyde- irinotecan hydrochloride injection, powder, for solution".DailyMed.20 October 2017.Retrieved25 May2020.
- ^abc"Onivyde pegylated liposomal EPAR".European Medicines Agency(EMA).25 October 2016.Retrieved25 May2020.Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^abcdefghijk"Irinotecan Hydrochloride".The American Society of Health-System Pharmacists.Archivedfrom the original on 22 December 2016.Retrieved8 December2016.
- ^British national formulary: BNF 69(69 ed.). British Medical Association. 2015. p. 624.ISBN9780857111562.
- ^World Health Organization(2023).The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023).Geneva: World Health Organization.hdl:10665/371090.WHO/MHP/HPS/EML/2023.02.
- ^Heinrich M, Barnes J, Gibbons S, Williamson EM (2012).Fundamentals of pharmacognosy and phytotherapy(2nd ed.). Edinburgh: Elsevier. p. 130.ISBN978-0-7020-5231-6.OCLC802051297.
- ^Guo Y, Shi M, Shen X, Yang C, Yang L, Zhang J (June 2014). "Capecitabine plus irinotecan versus 5-FU/leucovorin plus irinotecan in the treatment of colorectal cancer: a meta-analysis".Clinical Colorectal Cancer.13(2): 110–118.doi:10.1016/j.clcc.2013.12.004.PMID24461997.
- ^Kotaka M, Xu R, Muro K, Park YS, Morita S, Iwasa S, et al. (December 2016)."Study protocol of the Asian XELIRI ProjecT (AXEPT): a multinational, randomized, non-inferiority, phase III trial of second-line chemotherapy for metastatic colorectal cancer, comparing the efficacy and safety of XELIRI with or without bevacizumab versus FOLFIRI with or without bevacizumab".Chinese Journal of Cancer.35(1): 102.doi:10.1186/s40880-016-0166-3.PMC5178089.PMID28007025.
- ^abcdef"FDA approves irinotecan liposome for first-line treatment of metastatic pancreatic adenocarcinoma".U.S.Food and Drug Administration(FDA).13 February 2024.Retrieved19 February2024.This article incorporates text from this source, which is in thepublic domain.
- ^ab"Onivyde: EPAR – Product Information"(PDF).European Medicines Agency.25 October 2016.Archived(PDF)from the original on 16 January 2017.
- ^abHaberfeld H, ed. (2021).Austria-Codex(in German). Vienna: Österreichischer Apothekerverlag. Irinotecan Kabi 20 mg/ml Konzentrat zur Herstellung einer Infusionslösung.
- ^abIrinotecanMonograph.Accessed 18 September 2021.
- ^"Nirmala, M. Joyce, A. Samundeeswari, and P. Deepa Sankar. 2011." Natural Plant Resources in Anti-Cancer Therapy-A Review. "Research in Plant Biology 1 (3): 1–14".
- ^abPommier Y (January 2013)."Drugging topoisomerases: lessons and challenges".ACS Chemical Biology.8(1): 82–95.doi:10.1021/cb300648v.PMC3549721.PMID23259582.
- ^abReyhanoglu G, Smith T (2021)."Irinotecan".StatPearls.Treasure Island (FL): StatPearls Publishing.PMID32119328.Retrieved14 June2021.
- ^abcdefghijklmnopqrde Man FM, Goey AK, van Schaik RH, Mathijssen RH, Bins S (October 2018)."Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics".Clinical Pharmacokinetics.57(10): 1229–1254.doi:10.1007/s40262-018-0644-7.PMC6132501.PMID29520731.
- ^abcdefghKciuk M, Marciniak B, Kontek R (July 2020)."Irinotecan-Still an Important Player in Cancer Chemotherapy: A Comprehensive Overview".International Journal of Molecular Sciences.21(14): 4919.doi:10.3390/ijms21144919.PMC7404108.PMID32664667.
- ^abInnocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, et al. (April 2004)."Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan".Journal of Clinical Oncology.22(8): 1382–1388.doi:10.1200/JCO.2004.07.173.PMID15007088.
- ^O'Dwyer PJ, Catalano RB (October 2006)."Uridine diphosphate glucuronosyltransferase (UGT) 1A1 and irinotecan: practical pharmacogenomics arrives in cancer therapy".Journal of Clinical Oncology.24(28): 4534–4538.doi:10.1200/JCO.2006.07.3031.PMID17008691.
- ^"Camptosar: FDA-Approved Drugs".U.S.Food and Drug Administration(FDA).Retrieved25 May2020.
- ^"Drug Approval Package: Camptosar (Irinotecan Hydrochloride) NDA# 20-571/S-008".U.S.Food and Drug Administration(FDA).Retrieved25 May2020.
- ^"New Cancer Drug Approved".The New York Times.18 June 1996.Archivedfrom the original on 31 May 2016.Retrieved1 September2017.
- ^Temple R (22 October 1998)."New Drug Application"(PDF).U.S.Food and Drug Administration(FDA).Letter to Walker JS.Archived(PDF)from the original on 30 January 2012.Retrieved26 July2011.
- ^"Onivyde".U.S.Food and Drug Administration(FDA).29 September 2016.Retrieved25 May2020.
- ^News Release (22 October 2015)."FDA approves new treatment for advanced pancreatic cancer"(Press release). U.S.Food and Drug Administration(FDA). Archived fromthe originalon 24 October 2015.
Further reading
edit- Dean L (2015)."Irinotecan Therapy and UGT1A1 Genotype".In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.).Medical Genetics Summaries.National Center for Biotechnology Information(NCBI).PMID28520360.Bookshelf ID: NBK294473.