Monastrolis a cell-permeable small molecule inhibitor discovered by Thomas U. Mayer in the lab ofTim Mitchison.Monastrol was shown to inhibit thekinesin-5 (also known asKIF11,Kinesin Eg5), amotor proteinimportant for spindle bipolarity.[1]
| |

| |
| Names | |
|---|---|
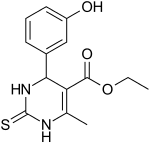
| IUPAC name
ethyl 4-(3-hydroxyphenyl)-6-methyl-2-sulfanylidene-3,4-dihydro-1H-pyrimidine-5-carboxylate
| |
| Other names
Monastrol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChemCID
|
|
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C14H16N2O3S | |
| Molar mass | 292.35344 |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Mechanism of action
editMonastrol binds to a long loop that is specific to the Eg5 (also known asKIF11or kinesin-5) kinesin family, and allosterically inhibits ATPase activity of the kinesin[2]
References
edit- ^Thomas U. Mayer; Tarun M. Kapoor; Stephen J. Haggarty; Randall W. King; Stuart L. Schreiber; Timothy J. Mitchison (1999)."Small Molecule Inhibitor of Mitotic Spindle Bipolarity Identified in a Phenotype-Based Screen".Science.286(5441): 971–974.doi:10.1126/science.286.5441.971.PMID10542155.S2CID15348455.
- ^Maliga Z, Kapoor TM, Mitchison TJ (September 2002)."Evidence that monastrol is an allosteric inhibitor of the mitotic kinesin Eg5".Chem. Biol.9(9): 989–96.doi:10.1016/S1074-5521(02)00212-0.PMID12323373.