Orphenadrine(sold under manybrand names)[1]is ananticholinergicdrugof theethanolamineantihistamineclass; it is closely related todiphenhydramine.It is amuscle relaxantthat is used to treat muscle pain and to help with motor control inParkinson's disease,but has largely been superseded by newer drugs.[citation needed]It is considered adirty drugdue to its multiple mechanisms of action in different pathways.[citation needed]It was discovered and developed in the 1940s.
 | |
| Clinical data | |
|---|---|
| Trade names | Generic; many brand names[1] |
| AHFS/Drugs | Monograph |
| MedlinePlus | a682162 |
| Pregnancy category |
|
| Routes of administration | Oral,intravenous,intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokineticdata | |
| Bioavailability | 90% |
| Protein binding | 95% |
| Metabolism | Hepaticdemethylation |
| Eliminationhalf-life | 13–20 hours[2] |
| Excretion | Renaland biliary |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.001.372 |
| Chemical and physical data | |
| Formula | C18H23NO |
| Molar mass | 269.388g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Medical use
editOrphenadrine is a skeletal muscle relaxant.[1][3]It is used to relieve pain caused by muscle injuries such asstrainsandsprains,in combination with rest and physical therapy.[3]A 2004 review found fair evidence that orphenadrine is effective for acute back or neck pain, but found insufficient evidence to establish the relative efficacy of the drug in relation to other drugs in the study.[4]
Orphenadrine and other muscle relaxants are sometimes used to treat pain arising fromrheumatoid arthritisbut there is no evidence they are effective for that purpose.[5]
In 2003, aCochrane Reviewof the use ofanticholinergicdrugs to improve motor function inParkinson's diseasefound that as a class, the drugs are useful for that purpose; it identified one single-site randomised, cross-over study of orphenadrine vs placebo.[6]Although orphenadrine and other anticholinergics have largely been superseded by other drugs; they have a use in alleviating motor function symptoms, and appear to help about 20% of people with Parkinson's.[7]
Side effects
editOrphenadrine has the side effects of the other common antihistamines in large part. Stimulation is somewhat more common than with other related antihistamines, and is especially common in the elderly. Common side effects include dry mouth, dizziness, drowsiness, constipation, urine retention, blurred vision, and headache.[3]Its use in Parkinson's is especially limited by these factors.[6]
Orphenadrine is contraindicated in patients withglaucoma,myasthenia gravis,sphincter relaxation disorders,digestive problems such aspeptic ulcers,bowel obstruction,or withenlarged prostate,bladder disorders;that is, they should not consume this drug.[8]
Continuous and/or cumulative use ofanticholinergicmedications, including first-generation antihistamines, is associated with higher risk of cognitive decline and dementia in older people.[9][10]
Pharmacology
editOrphenadrine is known to have these pharmacological properties:
- NonselectivemACh receptorantagonist(anticholinergic,58% as potent as atropine)[11]Various monographs and package inserts, nursing manuals, journal articles and so forth have proposed the theory that this anticholinergic (atropine-like) activity, NMDA antagonism and possible local anaesthetic and miscellaneous analgesic effects may be the reason for orphenadrine's efficacy against muscle and other pain.[12]These reasons are behind the use of orphenadrine and other drugs of a number of types which are used with paracetamol, aspirin, naproxen, and similar agents with or without opioid analgesics to more effectively manage pain of various types.[13]
- H1receptorantagonist(antihistamine)[13]
- NMDA receptor antagonist[14](Kivalue of6.0 ± 0.7 μM,one hundred times less potent thanphencyclidine,which binds with a Kiof 59 nM)[15][16]
- NDRI (norepinephrine and dopamine reuptake inhibitor)[17][18]
- Nav1.7,Nav1.8,andNav1.9sodium channel blocker[19]
- HERGpotassium channel blocker[20]
History
editGeorge Rieveschlwas a professor of chemistry at theUniversity of Cincinnatiand led a research program working onantihistamines.In 1943, one of his students, Fred Huber, synthesizeddiphenhydramine.Rieveschl worked withParke-Davisto test the compound, and the company licensed the patent from him. In 1947 Parke-Davis hired him as their Director of Research. While he was there, he led the development of orphenadrine, an analog of diphenhydramine.[21]
Prior to the development ofamantadinein the late 1960s and then other drugs, anticholinergics like orphenadrine were the mainstay of Parkinson's treatment.[7]
Formulation
editOrphenadrine has been available as a citrate salt and a hydrochloride salt; in the US as of February 2016 the citrate form was available in tablets, extended release tablets, compounding powder and by injection for acute use in a hospital setting.[1][22]
Orphenadrine is often available mixed withaspirin,paracetamol/acetaminophen,ibuprofen,caffeine,and/orcodeine.[1]
The brand names Norflex and Norgesic are formulations of the citrate salt of orphenadrine and Disipal is the hydrochloride salt.[23]
Chemistry
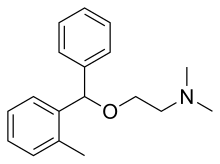
editOrphenadrine is aderivativeofdiphenhydraminewith amethyl groupadded to one of thephenyl rings.[24]
Stereochemistry
editOrphenadrine has achiral centerand twoenantiomers.When employed as a therapeutic agent, it is typically supplied as theracemate.[25]
| Enantiomers | |
|---|---|
(R)-orphenadrine CAS number: 33425-91-1 |
(S)-orphenadrine CAS number: 33425-89-7 |
References
edit- ^abcde"Orphenadrine".Drugs international listings.Retrieved5 February2016.
- ^Labout JJ, Thijssen C, Keijser GG, Hespe W (1982). "Difference between single and multiple dose pharmacokinetics of orphenadrine hydrochloride in man".European Journal of Clinical Pharmacology.21(4): 343–50.doi:10.1007/BF00637624.PMID7056281.S2CID24631265.
- ^abc"Orphenadrine".Medline Plus.1 December 2010.Retrieved6 February2016.
- ^Chou R, Peterson K, Helfand M (August 2004)."Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review".Journal of Pain and Symptom Management.28(2): 140–75.doi:10.1016/j.jpainsymman.2004.05.002.PMID15276195.
- ^Richards BL, Whittle SL, Buchbinder R (January 2012)."Muscle relaxants for pain management in rheumatoid arthritis".The Cochrane Database of Systematic Reviews.1:CD008922.doi:10.1002/14651858.CD008922.pub2.PMID22258993.S2CID205197256.
- ^abKatzenschlager R, Sampaio C, Costa J, Lees A (2003)."Anticholinergics for symptomatic management of Parkinson's disease".The Cochrane Database of Systematic Reviews.2002(2): CD003735.doi:10.1002/14651858.CD003735.PMC8728160.PMID12804486.
- ^abDonaldson I, Marsden CD, Schneider S (2012).Marsden's Book of Movement Disorders.Oxford University Press. p. 281.ISBN978-0-19-261911-2.
- ^"Orphenadrine Citrate Extended release label"(PDF).U.S. Food and Drug Administration.October 1998.Archived(PDF)from the original on 2023-10-04.Retrieved2023-10-04.
- ^Gray SL, Anderson ML, Dublin S, Hanlon JT,Hubbard R,Walker R, et al. (March 2015)."Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study".JAMA Internal Medicine.175(3): 401–407.doi:10.1001/jamainternmed.2014.7663.PMC4358759.PMID25621434.
- ^Carrière I, Fourrier-Reglat A, Dartigues JF, Rouaud O, Pasquier F, Ritchie K, Ancelin ML (July 2009)."Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study".Archives of Internal Medicine.169(14): 1317–1324.doi:10.1001/archinternmed.2009.229.PMC2933398.PMID19636034.
- ^Syvälahti EK, Kunelius R, Laurén L (February 1988). "Effects of antiparkinsonian drugs on muscarinic receptor binding in rat brain, heart and lung".Pharmacology & Toxicology.62(2): 90–4.doi:10.1111/j.1600-0773.1988.tb01852.x.PMID3353357.
- ^Nurses' Drug Guide 2010[full citation needed]
- ^abRumore MM, Schlichting DA (February 1985). "Analgesic effects of antihistaminics".Life Sciences.36(5): 403–16.doi:10.1016/0024-3205(85)90252-8.PMID2578597.
- ^Kornhuber J, Parsons CG, Hartmann S, Retz W, Kamolz S, Thome J, Riederer P (1995). "Orphenadrine is an uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist: binding and patch clamp studies".Journal of Neural Transmission. General Section.102(3): 237–46.doi:10.1007/BF01281158.PMID8788072.S2CID10142765.
- ^Kornhuber J, Parsons CG, Hartmann S, Retz W, Kamolz S, Thome J, Riederer P (1995). "Orphenadrine is an uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist: binding and patch clamp studies".Journal of Neural Transmission. General Section.102(3): 237–46.doi:10.1007/BF01281158.PMID8788072.S2CID10142765.
- ^Kapur S, Seeman P (2002)."NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia".Molecular Psychiatry.7(8): 837–44.doi:10.1038/sj.mp.4001093.PMID12232776.
- ^Pubill D, Canudas AM, Pallàs M, Sureda FX, Escubedo E, Camins A, Camarasa J (March 1999)."Assessment of the adrenergic effects of orphenadrine in rat vas deferens".The Journal of Pharmacy and Pharmacology.51(3): 307–12.doi:10.1211/0022357991772303.PMID10344632.S2CID31845784.
- ^Cheng MH, Block E, Hu F, Cobanoglu MC, Sorkin A, Bahar I (2015)."Insights into the Modulation of Dopamine Transporter Function by Amphetamine, Orphenadrine, and Cocaine Binding".Frontiers in Neurology.6:134.doi:10.3389/fneur.2015.00134.PMC4460958.PMID26106364.
- ^Desaphy JF, Dipalma A, De Bellis M, Costanza T, Gaudioso C, Delmas P, et al. (April 2009)."Involvement of voltage-gated sodium channels blockade in the analgesic effects of orphenadrine".Pain.142(3): 225–35.doi:10.1016/j.pain.2009.01.010.hdl:11586/128078.PMID19217209.S2CID17830280.
- ^Scholz EP, Konrad FM, Weiss DL, Zitron E, Kiesecker C, Bloehs R, et al. (December 2007). "Anticholinergic antiparkinson drug orphenadrine inhibits HERG channels: block attenuation by mutations of the pore residues Y652 or F656".Naunyn-Schmiedeberg's Archives of Pharmacology.376(4): 275–84.doi:10.1007/s00210-007-0202-6.PMID17965852.S2CID20049051.
- ^Sneader W (2005).Drug Discovery: A History.John Wiley & Sons. p. 405.ISBN978-0-471-89979-2.
- ^"FDA listing of Orphenadrine citrate registrations".United States Food and Drug Administration.Retrieved6 February2016.
- ^"Disipal Brand of Orphenadrine HCl".Riker.
- ^Morice C, Wermuth C (2015)."Ring Transformations. Chapter 9".InWermuth CG,Aldous D, Raboisson P, Rognan D (eds.).The Practice of Medicinal Chemistry(4th ed.). Elsevier. pp. 250–251.ISBN978-0-12-417213-5.
- ^Rote Liste Service GmbH (Hrsg.) (2017).Rote Liste 2017 Arzneimittelverzeichnis für Deutschland (einschließlich EU-Zulassungen und bestimmter Medizinprodukte).Vol. 57. Frankfurt/Main: Rote Liste Service GmbH. p. 207.ISBN978-3-946057-10-9.