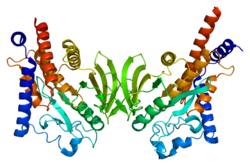
Protein tyrosine phosphatasenon-receptor type 22 (PTPN22) is acytoplasmaticproteinencoded bygenePTPN22and a member of PESTfamilyof protein tyrosine phosphatases. This protein is also called "PEST-domain Enriched Phosphatase" ( "PEP" ) or "Lymphoid phosphatase" ( "LYP" ). The name LYP is used strictly for thehumanprotein encoded byPTPN22,but the name PEP is used only for itsmousehomolog.However, both proteins have similar biological functions and show 70% identity inamino acidsequence. PTPN22 functions as a negative regulator ofT cell receptor(TCR)signaling,which maintainshomeostasisofT cellcompartment.[5][6]
Gene
editGenePTPN22is located on the p arm of the humanchromosome1. It is nearly 58 000base pairslong and contains 21exons.[7]In the case of mouse genome, it is located on the q arm of the chromosome 3. It is nearly 55 700 base pairs long and contains 23 exons.[8]
Structure
editPTPN22 is composed from 807 amino acids, and it weighs 91,705kDa.On itsN terminusit possessescatalyticdomain,which shows the highest level of conservation between human and mouse proteins. Other parts of PTPN22 are less conserved. After catalytic domain PTPN22 has approximately 300 amino acids long domain called "Interdomain". On theC terminusof PTPN22 there are 4proline-richmotifs(P1 - P4), which can mediateinteractionswith other proteins. P1 motif is the most important among them, because it is crucial for binding ofCSKkinase, andalleleencoding PTPN22 withmutatedP1 motif is associated with increased risk of numerousautoimmune diseases.[5]
Function
editRegulation of T cell receptor signaling
editA T cell receptor activation by a cognatepeptidetriggers a signaling pathway activating a T cell. The first event of this pathway is activation of theSRC family kinaseLCKby a dephosphorylation of its C terminal inhibitiontyrosine(Y505) and by a phosphorylation of its activation tyrosine (Y394).[9]LCK then phosphorylate tyrosines in theCD3 complexcreating a docking site for theSH2 domainof theSYK family kinaseZAP70,which is there phosphorylated too. The Phosphorylated ZAP70 then propagate a signal from a TCR, phosphorylating other proteins and creating a multi-protein complex, which activates downstream signaling pathways.[10]PTPN22 possess the ability todephosphorylateproteins included in proximal events of the TCR signaling and serves as an important negative regulator of a T cell activation. PTPN22 is able to bind the LCK with phosphorylated Y394, the phosphorylated ZAP70 and the phosphorylated ζ chain of CD3 complex. Thus, it binds molecules of a proximal TCR signaling only after their activation. PTPN22 can dephosphorylate those proteins and decrease the activating signal obtained by a T cell. Dephosphorylation of kinases LCK and ZAP70 by a PTPN22 is specific concerning the phosphorylated tyrosine in those proteins – only the Y394 of LCK and the Y493 of ZAP70 are dephosphorylated.[11]In the absence of PTPN22, an activated T cell receive a stronger activation signal, which is reflected by a greater influx ofCa2+cations into thecytosol,bigger phosphorylation of an LCK, ZAP70 and ERK and larger expansion of thosecells.[5][6][12][13][14][15]Theinhibitoryeffect on a TCR signaling was also verified with the usage of PTPN22 inhibitor on aJurkatT cell line and on human primary T cells,[14]and also with the experiments of PTPN22 overexpressionin vitro.[16][17]The expression of PTPN22 is upregulated after an activation of T cells and an antigen-experienced T cell have higher expression of PTPN22 than anaive T cell.[17][18]
The regulatory function of PTPN22 is particularly important during an activation by lowaffinitypeptides. In the absence of PTPN22, T cell cannot discriminate between strong and weakantigenssufficiently and those T cells become more responsive, which can be detected like increased upregulation oftranscription factorsandCD69,increasedERKphosphorylation,increased ability to expandin vivoand to producecytokines.Increased responsiveness can also break thetoleranceagainst low affinity self-antigens and is well visible, when PTPN22-deficient T cells get into alymphopenicenvironment.[15][19]
Regulation of regulatory T cells
editOne particular population of T cells, which is influenced by a PTPN22 deficiency is the population ofregulatory T cells(Treg cells). PTPN22-deficient mice contain higher amount of Treg cells inlymph nodesandspleensand this difference is more visible with increasing age of mice. There is also a change of the effector Treg cells: central Treg cells ratio in favor of the effector Treg cells. PTPN22 deficiency increases abilities of Treg cells to survive,differentiationof Treg cells from naive T cells, but not the ability toproliferatein vivo,and it also supports transition of central Treg cells to effector Treg cells.[13][20][21][22]One of the reasons, of the increased survival of PTPN22-deficient Treg cells, is that those cells have upregulated expression ofGITR,which increases their expansionin vivo.Treatment of PTPN22-deficient mice with an anti-GITR-L blockingantibodysuppresses the expansion of Treg cells.[20]PTPN22 deficiency does not impair the suppressive function of Treg cells. Actually there are some articles suggesting that PTPN22-deficient Treg cells possess an enhanced suppressive function or have a bigger ability to obtain an effector phenotype.[13][19][21]
Regulation of adhesiveness and motility
editNext to a TCR signaling PTPN22 regulates anadhesivenessand amotilityof T cells. PTPN22-deficient T cells have a prolonged interval of contact with anantigen presenting cell,which present a low affinity peptide. With a high affinity peptide the difference is not detectable. Part of the reason of the increased adhesiveness of those T cells is that enhanced TCR signaling results in a higher activation of theRAP1and a boosted inside-out signaling to activate the adhesive moleculeLFA-1.[15]In migrating T cells we can see the polarized localization of the PTPN22 at the leading edge of a migrating T cell, where it colocalizes with its substrates LCK and ZAP70. A downregulation of the PTPN22 increases motility, adhesivity and levels of phosphorylated LCK and phosphorylated ZAP70 in those cells. On the contrary, an overexpression of the PTPN22, but not the catalytically inactive PTPN22, increases motility of migrating T cells. An association of the PTPN22, but not its disease associated mutant form, with the LFA-1 results in a decreased LFA-1 clustering and a decreased adhesion.[23]The role of the PTPN22 in the regulation of LFA-1-mediated adhesion and motility is also supported by the observation of increased LFA-1 expression in PTPN22-/-Treg cells.[13]
Interaction partners
editThe C-terminal part of the PTPN22 bare proline-rich motifs providingbinding sitesfor putative interaction partners. One of those interaction partners is the cytoplasmatic tyrosine kinase CSK, which is a negative regulator of SRC family kinases and a TCR signaling as well as the PTPN22. CSK binds two prolin-rich motifs (P1 and P2) in the structure of PTPN22 through itsSH3 domainand the P1 motif is more important in this interaction. A deletion of the P1 motif greatly diminish the inhibitory effect of the PTPN22 on a TCR signaling. The Interaction of those enzymes is needed for their optimal function and the inhibition of TCR signaling.[16][24]It was also proposed that the interaction of PTPN22 and CSK regulate a localization of the PTPN22 and adissociationof this complex enables translocation of the PTPN22 tolipid raftsof aplasma membrane,where it can inhibit a TCR signaling. The mutant PTPN22, which is unable to bind CSK, is effectively recruited to a plasma membrane.[14]
Another interaction partner of the PTPN22 isTRAF3.This protein bind the PTPN22 and regulate its translocalization to a plasma membrane, in the absence of TRAF3 there is a bigger amount of the PTPN22 localized at a plasma membrane.[25]
Regulation of PTPN22
editIt was revealed that PTPN22 is regulated by a phosphorylation. PTPN22 is phosphorylated on theserinein the position 751 by the proteinPKC(most probably isoform PKCα) after activation of a T cell. This phosphorylation negatively regulates the TCR-suppressing function of the PTPN22. It also suppresses thepolyubiquitinationof PTPN22, which targets this protein fordegradation,and by this mean, it prolongshalf-lifeof the PTPN22. Phosphorylared PTPN22 interacts better with the CSK which hold PTPN22 away from a plasma membrane, where it can dephosphorylate proteins of a TCR signaling pathway. PTPN22 with the mutated serine 751 has shorter half-life, enhanced recruitment to plasma membrane and reduced interaction with CSK.[26]
PTPN22-deficient mice
editYoung PTPN22-deficient mice do not display any abnormality in peripherallymphoid organs,but older PTPN22-deficient mice (older than 6 months) develop asplen Omega lyand alymphadenopathy.In these older mice we can see an increased number of the T cells withphenotypeof the effector/memory T cells (CD44hi,CD62Llo), which have higherexpressionof the PTPN22 than naive T cells inWild Typemice. The expansion of those T cells is supported by the PTPN22 deficiency. A compartment of Treg cell is also influenced by the PTPN22 deficiencyin vivo.[12]Same as with the effector/memory T cells, PTPN22-deficient mice contain a bigger amount of Treg cells in lymph nodes and spleens and this difference is more visible with increasing age of mice. There is also a change of the effector Treg cells: central Treg cells ratio in favor of the effector Treg cells.[13][20][21][22]Influence of the PTPN22 deficiency on Treg cells number is consistent with the higher expression of PTPN22 in Treg cells than in naive T cells.[17]Another abnormality of PTPN22-deficient mice is a spontaneous formation of largegerminal centersin spleens andpeyer's patches.This formation of germinal centers is dependent on thecostimulationmoleculeCD40Land it is another consequence of the T cell dysregulation. PTPN22-deficient mice have increased levels of antibodies. However, there is no increase in levels ofautoantibodies.Despite those effects of the PTPN22 deficiency on a T cell compartment and an antibody production, PTPN22-deficient mice do not show signs of any autoimmune disease.[12][13]
Disease associated variant of PTPN22
editIn 2004, Bottini et al. discovered thesingle-nucleotide polymorphismin thePTPN22gene at nucleotide 1858. In this variant of the gene, normally occurringcytosineis substituted bythymine(C1858T). This cytosine encodes thecodonfor an amino acidargininein the position 620 of thelinear protein structure,but the mutation to thymine cause change of an arginine to atryptophan(R620W). The amino acid 620 is placed in the P1 motif, which is involved in the association with CSK and the mutation to tryptophan diminish the ability of the PTPN22 to bind CSK. The article reporting the existence of this variant also discovered that it is more frequent inDiabetes mellitus type 1patients.[27]The association of C1858T allele with type 1 diabetes was then confirmed by other studies.[28][29][30]In addition, C1858T allele ofPTPN22is associated with other autoimmune diseases includingRheumatoid arthritis,[31]systemic lupus erythematosus,juvenile idiopathic arthritis,[32]anti-neutrophil cytoplasmic antibody (ANCA)-associatedvasculitis,Graves’ disease,myasthenia gravis,Addison's disease.The contribution of the C1858TPTPN22allele to thosediseaseswas confirmed by more robustmeta-analysis.On the other hand, this allele is not linked to autoimmune diseases likemultiple sclerosis,Ulcerative colitis,pephigus vulgarisand others.[33][34][6]The exact way how the function of the PTPN22 is influenced by this mutation is still unknown. Throughout past years there were appearing evidences supporting that C1858T mutation is a loss-of-function mutation, but also evidences supporting that it is a gain-of-function mutation.[6][5]
References
edit- ^abcGRCh38: Ensembl release 89: ENSG00000134242–Ensembl,May 2017
- ^abcGRCm38: Ensembl release 89: ENSMUSG00000027843–Ensembl,May 2017
- ^"Human PubMed Reference:".National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^"Mouse PubMed Reference:".National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^abcdBottini N, Peterson EJ (2014-03-21)."Tyrosine phosphatase PTPN22: multifunctional regulator of immune signaling, development, and disease".Annual Review of Immunology.32(1): 83–119.doi:10.1146/annurev-immunol-032713-120249.PMC6402334.PMID24364806.
- ^abcdTizaoui K, Terrazzino S, Cargnin S, Lee KH, Gauckler P, Li H, et al. (June 2021). "The role of PTPN22 in the pathogenesis of autoimmune diseases: A comprehensive review".Seminars in Arthritis and Rheumatism.51(3): 513–522.doi:10.1016/j.semarthrit.2021.03.004.PMID33866147.S2CID233300534.
- ^"Chromosome 1: 113,813,811-113,871,759 - Region in detail - Homo sapiens - Ensembl genome browser 89".may2017.archive.ensembl.org.Retrieved2022-01-26.
- ^"Gene: Ptpn22 (ENSMUSG00000027843) - Summary - Mus musculus - Ensembl genome browser 89".may2017.archive.ensembl.org.Retrieved2022-01-26.
- ^Palacios EH, Weiss A (October 2004). "Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation".Oncogene.23(48): 7990–8000.doi:10.1038/sj.onc.1208074.PMID15489916.S2CID20109652.
- ^Courtney AH, Lo WL, Weiss A (February 2018)."TCR Signaling: Mechanisms of Initiation and Propagation".Trends in Biochemical Sciences.43(2): 108–123.doi:10.1016/j.tibs.2017.11.008.PMC5801066.PMID29269020.
- ^Wu J, Katrekar A, Honigberg LA, Smith AM, Conn MT, Tang J, et al. (April 2006)."Identification of substrates of human protein-tyrosine phosphatase PTPN22".The Journal of Biological Chemistry.281(16): 11002–11010.doi:10.1074/jbc.M600498200.PMID16461343.
- ^abcHasegawa K, Martin F, Huang G, Tumas D, Diehl L, Chan AC (January 2004). "PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells".Science.303(5658): 685–689.Bibcode:2004Sci...303..685H.doi:10.1126/science.1092138.PMID14752163.S2CID35540578.
- ^abcdefBrownlie RJ, Miosge LA, Vassilakos D, Svensson LM, Cope A, Zamoyska R (November 2012)."Lack of the phosphatase PTPN22 increases adhesion of murine regulatory T cells to improve their immunosuppressive function".Science Signaling.5(252): ra87.doi:10.1126/scisignal.2003365.PMC5836999.PMID23193160.
- ^abcVang T, Liu WH, Delacroix L, Wu S, Vasile S, Dahl R, et al. (March 2012)."LYP inhibits T-cell activation when dissociated from CSK".Nature Chemical Biology.8(5): 437–446.doi:10.1038/nchembio.916.PMC3329573.PMID22426112.
- ^abcSalmond RJ, Brownlie RJ, Morrison VL, Zamoyska R (September 2014)."The tyrosine phosphatase PTPN22 discriminates weak self peptides from strong agonist TCR signals".Nature Immunology.15(9): 875–883.doi:10.1038/ni.2958.PMC4148831.PMID25108421.
- ^abCloutier JF, Veillette A (January 1999)."Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase".The Journal of Experimental Medicine.189(1): 111–121.doi:10.1084/jem.189.1.111.PMC1887684.PMID9874568.
- ^abcPerry DJ, Peters LD, Lakshmi PS, Zhang L, Han Z, Wasserfall CH, et al. (August 2021)."Overexpression of thePTPN22Autoimmune Risk Variant LYP-620W Fails to Restrain Human CD4+T Cell Activation ".Journal of Immunology.207(3): 849–859.doi:10.4049/jimmunol.2000708.PMC8323970.PMID34301848.
- ^Salmond RJ, Brownlie RJ, Zamoyska R (2015-03-04)."Multifunctional roles of the autoimmune disease-associated tyrosine phosphatase PTPN22 in regulating T cell homeostasis".Cell Cycle.14(5): 705–711.doi:10.1080/15384101.2015.1007018.PMC4671365.PMID25715232.
- ^abKnipper JA, Wright D, Cope AP, Malissen B, Zamoyska R (2020-01-28)."PTPN22 Acts in a Cell Intrinsic Manner to Restrict the Proliferation and Differentiation of T Cells Following Antibody Lymphodepletion".Frontiers in Immunology.11:52.doi:10.3389/fimmu.2020.00052.PMC6997546.PMID32047502.
- ^abcNowakowska DJ, Kissler S (March 2016)."Ptpn22 Modifies Regulatory T Cell Homeostasis via GITR Upregulation".Journal of Immunology.196(5): 2145–2152.doi:10.4049/jimmunol.1501877.PMC4761465.PMID26810223.
- ^abcMaine CJ, Hamilton-Williams EE, Cheung J, Stanford SM, Bottini N, Wicker LS, Sherman LA (June 2012)."PTPN22 alters the development of regulatory T cells in the thymus".Journal of Immunology.188(11): 5267–5275.doi:10.4049/jimmunol.1200150.PMC3358490.PMID22539785.
- ^abZheng P, Kissler S (March 2013)."PTPN22 silencing in the NOD model indicates the type 1 diabetes-associated allele is not a loss-of-function variant".Diabetes.62(3): 896–904.doi:10.2337/db12-0929.PMC3581188.PMID23193190.
- ^Burn GL, Cornish GH, Potrzebowska K, Samuelsson M, Griffié J, Minoughan S, et al. (October 2016)."Superresolution imaging of the cytoplasmic phosphatase PTPN22 links integrin-mediated T cell adhesion with autoimmunity"(PDF).Science Signaling.9(448): ra99.doi:10.1126/scisignal.aaf2195.PMID27703032.S2CID3901060.
- ^Cloutier JF, Veillette A (September 1996)."Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells".The EMBO Journal.15(18): 4909–4918.doi:10.1002/j.1460-2075.1996.tb00871.x.PMC452228.PMID8890164.
- ^Wallis AM, Wallace EC, Hostager BS, Yi Z, Houtman JC, Bishop GA (May 2017)."TRAF3 enhances TCR signaling by regulating the inhibitors Csk and PTPN22".Scientific Reports.7(1): 2081.Bibcode:2017NatSR...7.2081W.doi:10.1038/s41598-017-02280-4.PMC5437045.PMID28522807.
- ^Yang S, Svensson MN, Harder NH, Hsieh WC, Santelli E, Kiosses WB, et al. (March 2020)."PTPN22 phosphorylation acts as a molecular rheostat for the inhibition of TCR signaling".Science Signaling.13(623): eaaw8130.doi:10.1126/scisignal.aaw8130.PMC7263369.PMID32184287.
- ^Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. (April 2004)."A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes".Nature Genetics.36(4): 337–338.doi:10.1038/ng1323.PMID15004560.S2CID40233008.
- ^Santiago JL, Martínez A, de la Calle H, Fernández-Arquero M, Figueredo MA, de la Concha EG, Urcelay E (August 2007)."Susceptibility to type 1 diabetes conferred by the PTPN22 C1858T polymorphism in the Spanish population".BMC Medical Genetics.8(1): 54.doi:10.1186/1471-2350-8-54.PMC1976418.PMID17697317.
- ^Zheng W, She JX (March 2005)."Genetic association between a lymphoid tyrosine phosphatase (PTPN22) and type 1 diabetes".Diabetes.54(3): 906–908.doi:10.2337/diabetes.54.3.906.PMID15734872.
- ^Chagastelles PC, Romitti M, Trein MR, Bandinelli E, Tschiedel B, Nardi NB (August 2010). "Association between the 1858T allele of the protein tyrosine phosphatase nonreceptor type 22 and type 1 diabetes in a Brazilian population".Tissue Antigens.76(2): 144–148.doi:10.1111/j.1399-0039.2010.01480.x.PMID20331840.
- ^Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, et al. (August 2004)."A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis".American Journal of Human Genetics.75(2): 330–337.doi:10.1086/422827.PMC1216068.PMID15208781.
- ^Hinks A, Barton A, John S, Bruce I, Hawkins C, Griffiths CE, et al. (June 2005). "Association between the PTPN22 gene and rheumatoid arthritis and juvenile idiopathic arthritis in a UK population: further support that PTPN22 is an autoimmunity gene".Arthritis and Rheumatism.52(6): 1694–1699.doi:10.1002/art.21049.PMID15934099.
- ^Zheng J, Ibrahim S, Petersen F, Yu X (December 2012)."Meta-analysis reveals an association of PTPN22 C1858T with autoimmune diseases, which depends on the localization of the affected tissue".Genes and Immunity.13(8): 641–652.doi:10.1038/gene.2012.46.PMID23076337.S2CID21331219.
- ^Tizaoui K, Kim SH, Jeong GH, Kronbichler A, Lee KS, Lee KH, Shin JI (March 2019)."Association of PTPN22 1858C/T Polymorphism with Autoimmune Diseases: A Systematic Review and Bayesian Approach".Journal of Clinical Medicine.8(3): 347.doi:10.3390/jcm8030347.PMC6462981.PMID30871019.
Further reading
edit- Gregersen PK (April 2005). "Pathways to gene identification in rheumatoid arthritis: PTPN22 and beyond".Immunological Reviews.204:74–86.doi:10.1111/j.0105-2896.2005.00243.x.PMID15790351.S2CID30275010.
- Brand O, Gough S, Heward J (October 2005). "HLA, CTLA-4 and PTPN22: the shared genetic master-key to autoimmunity?".Expert Reviews in Molecular Medicine.7(23): 1–15.doi:10.1017/S1462399405009981.PMID16229750.S2CID841442.
- Bottini N, Vang T, Cucca F, Mustelin T (August 2006). "Role of PTPN22 in type 1 diabetes and other autoimmune diseases".Seminars in Immunology.18(4): 207–213.doi:10.1016/j.smim.2006.03.008.PMID16697661.
- Gjörloff-Wingren A, Saxena M, Han S, Wang X, Alonso A, Renedo M, et al. (August 2000). "Subcellular localization of intracellular protein tyrosine phosphatases in T cells".European Journal of Immunology.30(8): 2412–2421.doi:10.1002/1521-4141(2000)30:8<2412::AID-IMMU2412>3.0.CO;2-J.PMID10940933.S2CID8132613.
- Hill RJ, Zozulya S, Lu YL, Ward K, Gishizky M, Jallal B (March 2002)."The lymphoid protein tyrosine phosphatase Lyp interacts with the adaptor molecule Grb2 and functions as a negative regulator of T-cell activation".Experimental Hematology.30(3): 237–244.doi:10.1016/S0301-472X(01)00794-9.PMID11882361.
- Chien W, Tidow N, Williamson EA, Shih LY, Krug U, Kettenbach A, et al. (July 2003)."Characterization of a myeloid tyrosine phosphatase, Lyp, and its role in the Bcr-Abl signal transduction pathway".The Journal of Biological Chemistry.278(30): 27413–27420.doi:10.1074/jbc.M304575200.PMID12764153.
- Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. (April 2004)."A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes".Nature Genetics.36(4): 337–338.doi:10.1038/ng1323.PMID15004560.
- Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, et al. (August 2004)."A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis".American Journal of Human Genetics.75(2): 330–337.doi:10.1086/422827.PMC1216068.PMID15208781.
- Kyogoku C, Langefeld CD, Ortmann WA, Lee A, Selby S, Carlton VE, et al. (September 2004)."Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE".American Journal of Human Genetics.75(3): 504–507.doi:10.1086/423790.PMC1182029.PMID15273934.
- Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, Howson JM, et al. (November 2004)."Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus".Diabetes.53(11): 3020–3023.doi:10.2337/diabetes.53.11.3020.PMID15504986.
- Ladner MB, Bottini N, Valdes AM, Noble JA (January 2005). "Association of the single nucleotide polymorphism C1858T of the PTPN22 gene with type 1 diabetes".Human Immunology.66(1): 60–64.doi:10.1016/j.humimm.2004.09.016.PMID15620463.
- Orozco G, Sánchez E, González-Gay MA, López-Nevot MA, Torres B, Cáliz R, et al. (January 2005)."Association of a functional single-nucleotide polymorphism of PTPN22, encoding lymphoid protein phosphatase, with rheumatoid arthritis and systemic lupus erythematosus".Arthritis and Rheumatism.52(1): 219–224.doi:10.1002/art.20771.PMID15641066.
- Steer S, Lad B, Grumley JA, Kingsley GH, Fisher SA (January 2005)."Association of R602W in a protein tyrosine phosphatase gene with a high risk of rheumatoid arthritis in a British population: evidence for an early onset/disease severity effect".Arthritis and Rheumatism.52(1): 358–360.doi:10.1002/art.20737.PMID15641088.
- Zheng W, She JX (March 2005)."Genetic association between a lymphoid tyrosine phosphatase (PTPN22) and type 1 diabetes".Diabetes.54(3): 906–908.doi:10.2337/diabetes.54.3.906.PMID15734872.
- Zhernakova A, Eerligh P, Wijmenga C, Barrera P, Roep BO, Koeleman BP (September 2005)."Differential association of the PTPN22 coding variant with autoimmune diseases in a Dutch population".Genes and Immunity.6(6): 459–461.doi:10.1038/sj.gene.6364220.PMID15875058.
External links
editOverview of all the structural information available in thePDBforUniProt:Q9Y2R2(Tyrosine-protein phosphatase non-receptor type 22) at thePDBe-KB.