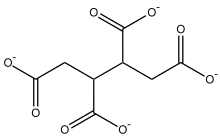
Polycarboxylatesareorganic compoundswith severalcarboxylic acidgroups. Butane-1,2,3,4-tetracarboxylate is one example. Often, polycarboxylate refers to linearpolymerswith a highmolecular mass(Mr≤ 100 000) and with manycarboxylategroups. They arepolymers of acrylic acidorcopolymersofacrylic acidandmaleic acid.Thepolymeris used as thesodium salt(see:sodium polyacrylate).[1]
Use
editPolycarboxylates are used asbuildersin detergents.[2]Their highchelatingpower, even at low concentrations, reducesdepositson the laundry and inhibits the crystal growth ofcalcite.
Polycarboxylate ethers (PCE) are used assuperplasticizersin concrete production.[3]
Safety
editPolycarboxylates are poorlybiodegradablebut have a lowecotoxicity.In thesewage treatment plant,the polymer remains largely in the sludge and is separated from thewastewater.
Polyamino acidslikepolyaspartic acidandpolyglutamic acidhave betterbiodegradabilitybut lower chelating performance than polyacrylates. They are also less stable towards heat and alkali. Since they contain nitrogen, they contribute toeutrophication.[4]
See also
editReferences
edit- ^Entry onPolycarboxylate.at:Römpp Online.Georg Thieme Verlag, retrieved 17. August 2016.
- ^Polycarboxylates(PDF),The Soap and Detergent Association,1996,retrieved2013-09-23
- ^Susanne Palecki (2006),Hochleistungsbeton unter Frost-Tau-Wechselbelastung: Schädigungs- und Transportmechanismen,p. 20,ISBN978-3-86537-725-8
- ^Yangxin Yu; Jin Zhao; Andrew E. Bayly (2008),"Development of Surfactants and Builders in Detergent Formulations",Chinese Journal of Chemical Engineering,16(4): 517–527,doi:10.1016/S1004-9541(08)60115-9