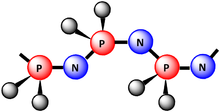
Polyphosphazenesinclude a wide range of hybridinorganic-organicpolymerswith a number of differentskeletal architectureswith the backboneP-N-P-N-P-N-.[1]In nearly all of these materials two organic side groups are attached to eachphosphoruscenter. Linear polymers have the formula (N=PR1R2)n,where R1and R2are organic (see graphic). Other architectures are cyclolinear and cyclomatrix polymers in which smallphosphazene ringsare connected together by organic chain units. Other architectures are available, such asblock copolymer,star,dendritic,orcomb-typestructures. More than 700 different polyphosphazenes are known, with different side groups (R) and different molecular architectures. Many of these polymers were first synthesized and studied in the research group ofHarry R. Allcock.[1][2][3][4][5]
Synthesis
editThe method ofsynthesisdepends on the type of polyphosphazene. The most widely used method for linear polymers is based on a two-step process.[1][2][3][4]In the first step,hexachlorocyclotriphosphazene(NPCl2)3is heated in a sealed system at 250 °C to convert it to a long chain linear polymer with typically 15,000 or morerepeating units.In the second step thechlorineatoms linked to phosphorus in the polymer are replaced by organic groups through reactions withalkoxides,aryloxides,aminesororganometallicreagents. Because many differentreagentscan participate in thismacromolecularsubstitution reaction,and because two or more reagents may be used, a large number of different polymers can be produced.. Variations to this process are possible usingpoly(dichlorophosphazene)made bycondensation reactions.[6]
Another synthetic process uses Cl3PNSiMe3as a precursor:[7]
- n Cl3PNSiMe3--> [Cl2PN]n+ ClSiMe3
Because the process is aliving cationic polymerization,block copolymers or comb, star, or dendritic architectures are possible.[8][9]Other synthetic methods include the condensation reactions of organic-substituted phosphoranimines.[10][11][12][13]
Cyclomatrix type polymers made by linking small molecule phosphazene rings together employ difunctional organic reagents to replace the chlorine atoms in (NPCl2)3,or the introduction ofallylorvinylsubstituents,which are thenpolymerizedbyfree-radicalmethods.[14]Such polymers may be useful as coatings orthermosettingresins,often prized for their thermal stability.
Properties and uses
editThe linear high polymers have thegeometryshown in the picture. More than 700 differentmacromoleculesthat correspond to e group]]s or combinations of different side groups. In these polymers the properties are defined by the high flexibility of thebackbone.Other potentially attractive properties include radiation resistance, highrefractive index,ultravioletandvisibletransparency, and itsfire resistance.The side groups exert an equal or even greater influence on the properties since they impart properties such ashydrophobicity,hydrophilicity,color,useful biological properties such asbioerodibility,orion transportproperties to the polymers. Representative examples of these polymers are shown below.
Thermoplastics
editThe first stablethermoplasticpoly(organophosphazenes), isolated in the mid 1960s byAllcock,Kugel, and Valan, were macromolecules with trifluoroethoxy,phenoxy,methoxy,ethoxy,or various amino side groups.[2][3][4]Of these early species, poly[bis(trifluoroethoxyphosphazene], [NP(OCH2CF3)2]n,has proved to be the subject of intense research due to itscrystallinity,high hydrophobicity, biological compatibility, fire resistance, general radiation stability, and ease of fabrication into films,microfibersandnanofibers.It has also been a substrate for varioussurface reactionsto immobilize biological agents. The polymers with phenoxy or amino side groups have also been studied in detail.
Phosphazene elastomers
editThe first large-scale commercial uses for linear polyphosphazenes were in the field of high technologyelastomers,with a typical example containing a combination of trifluoroethoxy and longer chain fluoroalkoxy groups.[15][16][17][18]The mixture of two different side groups eliminates thecrystallinityfound in single-substituent polymers and allows the inherent flexibility andelasticityto become manifest.Glass transitiontemperatures as low as -60 °C are attainable, and properties such as oil-resistance andhydrophobicityare responsible for their utility in land vehicles andaerospacecomponents. They have also been used in biostable biomedical devices.[19]
Otherside groups,such as non-fluorinated alkoxy oroligo-alkyl ether units, yield hydrophilic or hydrophobic elastomers with glass transitions over a broad range from -100 °C to 100 °C.[20]Polymers with two different aryloxy side groups have also been developed as elastomers for fire-resistance as well asthermalandsound insulationapplications.
Polymer electrolytes
editLinear polyphosphazenes witholigo-ethyleneoxyside chains are gums that are good solvents for salts such as lithiumtriflate.These solutions function aselectrolytesfor lithium ion transport, and they were incorporated into fire-resistantrechargeablelithium-ion polymer battery.[21][22][23]The same polymers are also of interest as the electrolyte indye-sensitized solar cells.[24]Other polyphosphazenes withsulfonatedaryloxy side groups are proton conductors of interest for use in the membranes ofproton exchange membrane fuel cells.[25]
Hydrogels
editWater-soluble poly(organophosphazenes) with oligo-ethyleneoxy side chains can becross-linkedbygamma-radiation.The cross-linked polymers absorb water to formhydrogels,which are responsive to temperature changes, expanding to a limit defined by the cross-link density below acritical solution temperature,but contracting above that temperature. This is the basis of controlled permeability membranes. Other polymers with both oligo-ethyleneoxy and carboxyphenoxy side groups expand in the presence ofmonovalentcationsbut contract in the presence of di- or tri-valent cations, which form ionic cross-links.[26][27][28][29][30]Phosphazene hydrogels have been utilized for controlled drug release and other medical applications.[27]
Bioerodible polyphosphazenes
editThe ease with which properties can be controlled and fine-tuned by the linkage of different side groups to polyphosphazene chains has prompted major efforts to addressbiomedicalmaterials challenges using these polymers.[31]Different polymers have been studied asmacromoleculardrug carriers,as membranes for thecontrolled delivery of drugs,as biostableelastomers,and especially as tailoredbioerodiblematerials for the regeneration of livingbone.[32][33][34][35]An advantage for this last application is that poly(dichlorophosphazene) reacts withamino acidethylesters(such as ethylglycinateor the corresponding ethyl esters of numerous other amino acids) through theaminoterminus to form polyphosphazenes with amino acid ester side groups. These polymershydrolyzeslowly to a near-neutral, pH-buffered solutionof the amino acid, ethanol, phosphate, and ammonium ion. The speed of hydrolysis depends on the amino acid ester, withhalf-livesthat vary from weeks to months depending on the structure of the amino acid ester.Nanofibersand porous constructs of these polymers assistosteoblastreplication and accelerate the repair of bone in animal model studies.
Commercial aspects
editNo applications are commercialized for polyphosphazenes. The cyclic trimerhexachlorophosphazene((NPCl2)3) is commercially available. It is the starting point for most commercial developments. High performanceelastomersknown as PN-F or Eypel-F have been manufactured for seals,O-rings,and dental devices. An aryloxy-substituted polymer has also been developed as a fire resistant expanded foam forthermalandsound insulation.The patent literature contains many references to cyclomatrix polymers derived from cyclic trimeric phosphazenes incorporated into cross-linked resins for fire resistantcircuit boardsand related applications.
References
edit- ^abcAllcock, H. R., Kugel, R. L. (1965). "Synthesis of High Polymeric Alkoxy and Aryloxyphosphonitriles".Journal of the American Chemical Society.87(18): 4216–4217.doi:10.1021/ja01096a056.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^abcAllcock, H. R., Kugel, R. L., Valan, K. J. (1966). "Phosphonitrilic Compounds. VI. High Molecular Weight Poly(alkoxy- and aryloxyphosphazenes)".Inorganic Chemistry.5(10): 1709–1715.doi:10.1021/ic50044a016.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^abcAllcock, H. R., Kugel, R. L. (1966). "Phosphonitrilic Compounds. VII. High Molecular Weight Poly(diaminophosphazenes)".Inorganic Chemistry.5(10): 1716–1718.doi:10.1021/ic50044a017.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^abc"Allcock Research Group Web Site".
- ^Allcock, Harry R. (2003).Chemistry and Applications of Polyphosphazenes.Wiley-Interscience.
- ^Gleria, M., De Jaeger, R. and Potin, P. (2004).Synthesis and Characterization of Poly(organophosphazenes).New York: Nova Science Publishers.
{{cite book}}:CS1 maint: multiple names: authors list (link) - ^Rothemund, Sandra; Teasdale, Ian (2016)."Preparation of polyphosphazenes: A tutorial review".Chemical Society Reviews.45(19): 5200–5215.doi:10.1039/C6CS00340K.PMC5048340.PMID27314867.
- ^Honeyman, C. H., Manners, I., Morrissey, C. T., Allcock, H. R. (1995). "Ambient Temperature Synthesis of Poly(dichlorophosphazene) with Molecular Weight Control".Journal of the American Chemical Society.117(26): 7035–7036.doi:10.1021/ja00131a040.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Allcock, H. R., Crane, C. A., Morrissey, C. T., Nelson, J. M., Reeves, S. D., Honeyman, C. H., Manners, I. (1996). ""Living" Cationic Polymerization of Phosphoranimines as an Ambient Temperature Route to Polyphosphazenes with Controlled Molecular Weights ".Macromolecules.29(24): 7740–7747.Bibcode:1996MaMol..29.7740A.doi:10.1021/ma960876j.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Wisian-Neilson, P.; Neilson, R. H. (1980). "Poly(dimethylphosphazene), (Me2PN)n".Journal of the American Chemical Society.102(8): 2848–2849.doi:10.1021/ja00528a060.
- ^Neilson, R. H., Wisian Neilson, P. (1988). "Poly(alkyl/arylphosphazenes) and their precursors".Chemical Reviews.88(3): 541–562.doi:10.1021/cr00085a005.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Montague, R. A., Matyjaszewski, K. (1990). "Synthesis of Poly[bis(trifluoroethoxy)phosphazene] under Mild Conditions using a Fluoride Initiator".Journal of the American Chemical Society.112(18): 6721–6723.doi:10.1021/ja00174a047.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Matyjaszewski, K., Moore, M. M., White (1993). "Synthesis of Polyphosphazene Block Copolymers bearing Alkoxyethoxy and Trifluoroethoxy Groups".Macromolecules.26(25): 6741–6748.Bibcode:1993MaMol..26.6741M.doi:10.1021/ma00077a008.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Allen, C. W., Shaw, J. C., Brown, D. E. (1988). "Copolymerization of (( Alpha -methylethenyl)phenyl)pentafluorocyclotriphosphazenes with Styrene and Methyl Methacrylate".Macromolecules.21(9): 2653–2657.Bibcode:1988MaMol..21.2653A.doi:10.1021/ma00187a001.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Rose, S. H. (1968). "Synthesis of Phosphonitrilic Fluoroelastomers".Journal of Polymer Science Part B: Polymer Letters.6(12): 837–839.Bibcode:1968JPoSL...6..837R.doi:10.1002/pol.1968.110061203.
- ^Singler, R. E., Schneider, N. S., Hagnauer, G. L. (1975). "Polyphosphazenes: Synthesis—properties—Applications".Polymer Engineering and Science.15(5): 321–338.doi:10.1002/pen.760150502.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^US 4945139,Charles H. Kolich; W. Dirk Klobucar & Jeffrey T. Books, "Process for surface treating phosphonitrilic fluoroelastomers", published Jul 31, 1990, assigned to Ethyl Corporation
- ^Tate, D. P. (1974). "Polyphosphazene Elastomers".Journal of Polymer Science: Polymer Symposia.48:33–45.doi:10.1002/polc.5070480106.
- ^Gettleman, L.; Farris, C. L.; Rawls, H. R. & LeBouef, R. J. (1984). "Soft and Firm Denture Liner for a Composite Denture and Method of Fabricating".
{{cite journal}}:Cite journal requires|journal=(help) - ^Weikel, Arlin L.; Lee, David K.; Krogman, Nicholas R.; Allcock, Harry R. (2011). "Phase changes of poly(alkoxyphosphazenes), and their behavior in the presence of oligoisobutylene".Polymer Engineering & Science.51(9): 1693–1700.doi:10.1002/pen.21623.
- ^Blonsky, P. M.; Shriver, D. F.; Austin, P. E.; Allcock, H. R. (1984)."Polyphosphazene solid electrolytes".Journal of the American Chemical Society.106(22): 6854–6855.doi:10.1021/ja00334a071.Archived fromthe originalon September 24, 2017.
- ^H. R.; O’Connor, S. J. M.; Olmeijer, D. L.; Napierala, M. E.; Cameron, C. G. (1996). "Cation Complexation and Conductivity in Crown Ether Bearing Polyphosphazenes".Macromolecules.29(23): 7544–7552.Bibcode:1996MaMol..29.7544A.doi:10.1021/ma960592z.
- ^Fei, S.-T.; Allcock, H. R. (2010). "Methoxyethoxyethoxyphosphazenes as Ionic Conductive Fire Retardant /additives for Lithium Battery Systems".Journal of Power Sources.195(7): 2082–2088.Bibcode:2010JPS...195.2082F.doi:10.1016/j.jpowsour.2009.09.043.
- ^Fei, S.-T; Lee, S.-H. A; Pursel, S. M.; Basham, J.; Hess, A.; Grimes, C. A.; Horn, M. W.; Mallouk, T. E.; Allcock, H. R. (2011). "Electrolyte Infiltration in Phosphazene-Based Dye-Sensitized Solar Cells".Journal of Power Sources.21(11): 2641–2651.Bibcode:2011JPS...196.5223F.doi:10.1016/j.jpowsour.2011.01.052.
- ^Tang, H.; Pintauro, P. N. (2001). "Polyphosphazene membranes. IV. Polymer morphology and proton conductivity in sulfonated poly[bis(3-methylphenoxy)phosphazene] films".Journal of Applied Polymer Science.79:49–59.doi:10.1002/1097-4628(20010103)79:1<49::aid-app60>3.0.co;2-j.
- ^H. R. Allcock; S. Kwon; G. H. Riding; R. J. Fitzpatrick; J. L. Bennett (1988). "Hydrophilic Polyphosphazenes as Hydrogels: Radiation Cr osslinking and Hydrogel Characteristics of Poly[bis(methoxyethoxyethoxy)phosphazene]".Biomaterials.9(6): 509–513.doi:10.1016/0142-9612(88)90046-4.PMID3224138.
- ^abKim, J.; Chun, C.; Kim, B.; Hong, J. M.; Cho J.–K; Lee. S. H. & Song, S.–C. (2012). "Thermosensitive/magnetic poly(organophosphazene) hydrogel as a long-term magnetic resonance contrast platform".Biomaterials.33(1): 218–224.doi:10.1016/j.biomaterials.2011.09.033.PMID21975461.
- ^H. R. Allcock; S. R. Pucher; M. L. Turner; R. J. Fitzpatrick (1992). "Poly(organophosphazenes) with Poly(alkyl ether) Side Groups: A Study of Their Water Solubility and the Swelling Characteristics of Their Hydrogels".Macromolecules.25(21): 5573–5577.Bibcode:1992MaMol..25.5573A.doi:10.1021/ma00047a002.
- ^.R. Allcock; R. J. Fitzpatrick; K. B. Visscher (1992). "Thin-layer grafts of poly[bis((methoxyethoxy)ethoxy)phosphazene] on organic polymer surfaces".Chemistry of Materials.4(4): 775–780.doi:10.1021/cm00022a007.
- ^H. R. Allcock; A. M. A. Ambrosio (1996). "Synthesis and Characterization of pH-Senstitive Poly(organophosphazene) Hydrogels".Biomaterials.17(23): 2295–2302.doi:10.1016/0142-9612(96)00073-7.PMID8968526.
- ^Chen, Feiyang; Teniola, Oyindamola R.; Laurencin, Cato T. (2022-04-01)."Biodegradable polyphosphazenes for regenerative engineering".Journal of Materials Research.37(8): 1417–1428.doi:10.1557/s43578-022-00551-z.ISSN2044-5326.PMC9531846.S2CID248257951.
- ^Allcock, H. R.; Pucher, S. R.; Scopelianos, A. G. (1994). "Poly[amino acid ester)phosphazenes] as Substrates for the Controlled Release of Small Molecules".Biomaterials.15(8): 563–569.doi:10.1016/0142-9612(94)90205-4.PMID7948574.
- ^Deng, M., Kumbar, S. G., Wan, Y. Toti, U. S. Allcock, H. R., Laurencin, C. T. (2010). "Polyphosphazene Polymers for Tissue Engineering: An Analysis of Material Synthesis, Characterization, and Applications".Soft Matter.6(14): 3119–3132.Bibcode:2010SMat....6.3119D.doi:10.1039/b926402g.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Deng, M., Kumbar, S. G., Nair, L. S. Arlin L. Weikel, A. L, Allcock, H. R., Laurencin, C. T. (2011). "Biomimetic Structures: Biological Implications of Dipeptide-Substituted Polyphosphazene–Polyester Blend Nanofiber Matrices for Load-Bearing Bone Regeneration".Advanced Functional Materials.21(14): 2641–2651.doi:10.1002/adfm.201100275.S2CID96953240.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Allcock, H. R.; Morozowich, N. (2012). "Bioerodible Polyphosphazenes and their Medical Potential".Polymer Chemistry.3(3): 578–590.doi:10.1039/c1py00468a.
Further information
edit"H. R. Allcock Research Group".Retrieved2020-08-22.