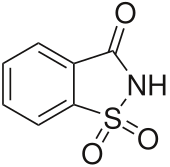
Saccharin,also calledsaccharine,benzosulfimide,orE954,or used in saccharin sodium or saccharin calcium forms, is a non-nutritiveartificial sweetener.[1][5]Saccharin is asultamthat is about 500 times sweeter thansucrose,but has a bitter or metallicaftertaste,especially at high concentrations.[1]It is used to sweeten products, such asdrinks,candies,baked goods,tobaccoproducts,excipients,and for masking the bitter taste of somemedicines.[1][5]It appears as white crystals and is odorless.[1]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1H-1λ6,2-Benzothiazole-1,1,3(2H)-trione | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.202 |
| E number | E954(glazing agents,...) |
| KEGG | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C7H5NO3S | |
| Molar mass | 183.18g·mol−1 |
| Appearance | White crystalline solid |
| Density | 0.828 g/cm3 |
| Melting point | 228.8 to 229.7 °C (443.8 to 445.5 °F; 501.9 to 502.8 K) |
| 1 g per 290 mL | |
| Acidity(pKa) | 1.6[4] |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Etymology
editSaccharin derives its name from the word "saccharine", meaning "sugary". The word saccharine is used figuratively, often in a derogative sense, to describe something "unpleasantly over-polite" or "overly sweet".[6]Both words are derived from theGreekwordσάκχαρον(sakkharon) meaning "gravel".[7]Similarly, saccharose is an obsolete name forsucrose(table sugar).
Properties
editSaccharin is heat-stable.[8]It does not react chemically with other food ingredients; as such, it stores well. Blends of saccharin with other sweeteners are often used to compensate for each sweetener's weaknesses and faults. A 10:1cyclamate–saccharin blend is common in countries where both these sweeteners are legal; in this blend, each sweetener masks the other's offtaste. Saccharin is often used withaspartamein dietcarbonated soft drinks,so some sweetness remains should the fountain syrup be stored beyond aspartame's relatively short shelf life.
In its acid form, saccharin is not water-soluble. The form used as an artificial sweetener is usually itssodiumsalt.[9]Thecalciumsalt is also sometimes used, especially by people restricting theirdietary sodiumintake. Both salts are highly water-soluble: 0.67 g/ml in water at room temperature.[10][11]
Safety and health effects
editIn the 1970s, studies performed on laboratory rats found an association between consumption of high doses of saccharin and the development ofbladder cancer.[12]However, further study determined that this effect was due to a mechanism that is not relevant to humans (deposition of crystals; see§ Historysection).[12]Epidemiologicalstudies have shown no evidence that saccharin is associated with bladder cancer in humans.[12][13]TheInternational Agency for Research on Cancer(IARC) originally classified saccharin inGroup 2B( "possibly carcinogenic to humans" ) based on the rat studies, but downgraded it toGroup 3( "not classifiable as to the carcinogenicity to humans" ) upon review of the subsequent research.[14]
Saccharin has nofood energyand no nutritional value.[15]It is safe to consume for individuals withdiabetesorprediabetes.[16][17]
People with sulfonamide allergies can experience allergic reactions to saccharin, although it has been suggested that this may be due to a general predisposition to allergic reactions rather than a specific cross-reaction between antimicrobial sulfonamides and non-antimicrobial ones (like saccharin).[18][19]
History
editSaccharin was produced first in 1879, byConstantin Fahlberg,a chemist working oncoal tarderivatives inIra Remsen's laboratory atJohns Hopkins University.[20]Fahlberg noticed asweet tasteon his hand one evening, and connected this with the compound benzoic sulfimide on which he had been working that day.[21][22]Fahlberg and Remsen published articles on benzoic sulfimide in 1879 and 1880.[10][23]In 1884, then working on his own inNew York City,Fahlberg applied for patents in several countries, describing methods of producing this substance that he named saccharin.[24]Two years later, he began production of the substance in a factory in a suburb ofMagdeburgin Germany. Fahlberg would soon grow wealthy, while Remsen merely grew irritated, believing he deserved credit for substances produced in his laboratory. On the matter, Remsen commented, "Fahlberg is a scoundrel. It nauseates me to hear my name mentioned in the same breath with him."[25]
Although saccharin was commercialized not long after its discovery, until sugar shortages duringWorld War I,its use had not become widespread. Its popularity further increased during the 1960s and 1970s among dieters, since saccharin is acalorie-free sweetener. In the United States, saccharin is often found in restaurants inpinkpackets; the most popular brand is "Sweet'n Low".
Due to the difficulty of importing sugar from theWest IndiesduringWorld War I,theBritish Saccharin Companywas founded in 1917 to produce saccharin at its Paragon Works nearAccrington,Lancashire.Production was licensed and controlled by theBoard of Tradein London. Production continued on the site until 1926.
Government regulation
editThe examples and perspective in this sectiondeal primarily with the United States and do not represent aworldwide viewof the subject.(October 2015) |
Starting in 1907, theUnited States Food and Drug Administrationbegan investigating saccharin as a result of thePure Food and Drug Act.Harvey Wiley,then the director of the bureau of chemistry for the FDA, viewed it as an illegal substitution of a valuable ingredient, sugar, by a less valuable ingredient. In a clash that had career consequences, Wiley told PresidentTheodore Roosevelt,"Everyone who ate that sweet corn was deceived. He thought he was eating sugar, when in point of fact he was eating a coal tar product totally devoid of food value and extremely injurious to health." But Roosevelt himself was a consumer of saccharin, and, in a heated exchange, Roosevelt angrily answered Wiley by stating, "Anybody who says saccharin is injurious to health is an idiot." The episode proved the undoing of Wiley's career.[26]
In 1911, Food Inspection Decision 135 stated that foods containing saccharin wereadulterated.[27]However, in 1912, Food Inspection Decision 142 stated that saccharin was not harmful.[28]
More controversy was stirred in 1969 with the discovery of files from the FDA's investigations of 1948 and 1949. These investigations, which had originally argued against saccharin use, were shown to prove little about saccharin being harmful to human health.[citation needed]In 1977, the FDA made an attempt to completely ban the substance,[11][29]following studies showing that the substance causedbladder cancerin rats. The attempted ban was unsuccessful due to public opposition that was encouraged by industry advertisements,[29]and instead the following label was mandated: "Use of this product may be hazardous to your health. This product contains saccharin which has been determined to cause cancer in laboratory animals". That requirement was dropped in 2000 following new research that concluded humans reacted differently than rats and were not at risk of cancer at typical intake levels.[29](See also:§ Warning label addition and removalbelow.) The sweetener has continued to be widely used in the United States and is now the third-most popular artificial sweetener behindsucraloseandaspartame.
In theEuropean Union,saccharin is also known by theE number(additive code) E954.[30]
The current status of saccharin is that it is allowed in most countries, and countries such as Canada have lifted their previous ban of it as a food additive.[31]The claims that it is associated with bladder cancer were shown to be unfounded in experiments on primates.[32](It is, however, prohibited to mail saccharin tablets or packets to France.)[33]
Saccharin was formerly on California's list of chemicals known to the state to cause cancer for the purposes ofProposition 65,but it was delisted in 2001.[34]
Warning label addition and removal
editIn 1958, the United States Congress amended theFood, Drugs, and Cosmetic Act of 1938with theDelaney clauseto mandate that theFood and Drug Administrationnot approve substances that "induce cancer in man, or, after tests, [are] found to induce cancer in animals." Studies in laboratory rats during the early 1970s linked saccharin with the development of bladder cancer in rodents. As a consequence, all food containing saccharin was labeled with a warning meeting the requirement of theSaccharin Study and Labeling Act of 1977.[35]
However, in 2000, the warning labels were removed because scientists learned that rodents, unlike humans, have a unique combination of high pH, highcalcium phosphate,and high protein levels in their urine.[36][37]One or more of the proteins that are more prevalent in male rats combine with calcium phosphate and saccharin to produce microcrystals that damage the lining of the bladder. Over time, the rat's bladder responds to this damage by overproducing cells to repair the damage, which leads to tumor formation. Since this does not occur in humans, there is no elevated risk of bladder cancer.[38]
The delisting of saccharin led to legislation repealing the warning label requirement for products containing saccharin.[39]In 2001, the U.S. Food and Drug Administration and the state of California reversed their positions on saccharin, declaring it safe for consumption.[29]The FDA's decision followed a 2000 determination by the U.S.Department of Health and Human Services'National Toxicology Programto remove saccharin from its list of carcinogens.
TheEnvironmental Protection Agencyhas officially removed saccharin and its salts from their list of hazardous constituents and commercial chemical products. In a release in December 2010, the EPA stated that saccharin is no longer considered a potential hazard to human health.[40]
Chemistry
editPreparation
editSaccharin can be produced in various ways.[41]The original route by Remsen and Fahlberg starts withtoluene;another route begins witho-chlorotoluene.[42]Sulfonation of toluene bychlorosulfonic acidgives theorthoandparasubstitutedsulfonyl chlorides.Theorthoisomer is separated and converted to thesulfonamidewithammonia.Oxidation of the methyl substituent gives the carboxylic acid, which cyclicizes to give saccharin free acid:[43]
In 1950, an improved synthesis was developed at theMaumee Chemical CompanyofToledo, Ohio.In this synthesis, themethyl anthranilatesuccessively reacts withnitrous acid(fromsodium nitriteandhydrochloric acid),sulfur dioxide,chlorine,and thenammoniato yield saccharin:[43]
Properties and reactions
editThe free acid of saccharin has a lowpKaof 1.6 (the acidic hydrogen being that attached to the nitrogen).[4]Saccharin can be used to prepare exclusively disubstitutedaminesfromalkyl halidesvia a nucleophilic substitution,[44]followed by aGabriel synthesis.[45][46]
See also
editReferences
edit- ^abcde"Saccharin".PubChem, US National Library of Medicine. 13 June 2023.Retrieved15 June2023.
- ^"Saccharin (CAS: 81-07-2)".Merck Millipore.2023.RetrievedAugust 22,2022.
- ^NCERT Chemistry Part II Textbook for Class XII.Delhi:NCERT.2021. p. 449.ISBN978-81-7450-716-7.
- ^abBell RP, Higginson WC (1949)."The Catalyzed Dehydration of Acetaldehyde Hydrate, and the Effect of Structure on the Velocity of Protolytic Reactions".Proceedings of the Royal Society A.197(1049):141–159.Bibcode:1949RSPSA.197..141B.doi:10.1098/rspa.1949.0055.
- ^ab"Saccharin".Drugs.16 August 2022.Retrieved15 June2023.
- ^"Saccharine".Reference.Archivedfrom the original on 2007-03-03.
- ^"Saccharine".etymonline.Archivedfrom the original on 2006-03-23.
- ^"Sweetener Comparisons".Food Ingredient Series.NCSU. 2006. Archived fromthe originalon 2019-01-20.
- ^Chattopadhyay S, Raychaudhuri U, Chakraborty R (April 2014)."Artificial sweeteners - a review".Journal of Food Science and Technology.51(4):611–21.doi:10.1007/s13197-011-0571-1.PMC3982014.PMID24741154.
- ^abFahlberg C, Remsen I (1879)."Über die Oxydation des Orthotoluolsulfamids"[On the oxidation of orthotoluenesulphamide].Berichte der Deutschen Chemischen Gesellschaft zu Berlin.12:469–473.doi:10.1002/cber.187901201135.Archivedfrom the original on 2013-05-13.
- ^abPriebe PM, Kauffman GB (1980). "Making governmental policy under conditions of scientific uncertainty: a century of controversy about saccharin in Congress and the laboratory".Minerva.18(4):556–74.doi:10.1007/BF01096124.PMID11611011.S2CID40526005.
- ^abc"Artificial Sweeteners and Cancer".National Cancer Institute.2005-08-18.Archivedfrom the original on 2015-12-08.
- ^Weihrauch MR, Diehl V (October 2004)."Artificial sweeteners--do they bear a carcinogenic risk?".Annals of Oncology.15(10):1460–5.doi:10.1093/annonc/mdh256.PMID15367404.
- ^"Saccharin: FDA Agencies".University of Minnesota,Environmental Health Sciences.Archivedfrom the original on 2016-02-27.
- ^"Common Terms: S–Z".American Diabetes Association.Archivedfrom the original on 2015-11-28.
- ^"Low-Calorie Sweeteners: What's News, What's New".American Diabetes Association.Archivedfrom the original on 2016-03-04.
- ^"Are Artificial Sweeteners Safe for People With Diabetes?".Cleveland Clinic.2015-06-29.Archivedfrom the original on 2016-10-02.
- ^Strom, Brian L.; Schinnar, Rita; Apter, Andrea J.; Margolis, David J.; Lautenbach, Ebbing; Hennessy, Sean; Bilker, Warren B.; Pettitt, Dan (2003)."Absence of Cross-Reactivity between Sulfonamide Antibiotics and Sulfonamide Nonantibiotics".New England Journal of Medicine.349(17):1628–1635.doi:10.1056/nejmoa022963.PMID14573734.
- ^Giles, Amber; Foushee, Jaime; Lantz, Evan; Gumina, Giuseppe (2019)."Sulfonamide Allergies".Pharmacy.7(3): 132.doi:10.3390/pharmacy7030132.PMC6789825.PMID31514363.
- ^(As discussed below, the relative contributions of Fahlberg and Remsen to the discovery were later contested, with no final resolution in sight; the 1879 paper announcing the discovery lists both names as authors, with Fahlberg's name first.)
- ^Fahlberg's account of how he discovered the sweetness of saccharin appears in:Anon. (July 17, 1886)."The inventor of saccharine".Scientific American.new series.60(3): 36. Archived fromthe originalon 2017-03-14.
- ^Myers RL, Myers RL (2007).The 100 Most Important Chemical Compounds: A Reference Guide.Westport, Connecticut: Greenwood Press. p. 241.ISBN978-0-313-33758-1.
- ^Remsen I, Fahlberg C (February 1880)."On the Oxidation of Substitution Products of Aromatic Hydrocarbons. IV. – On the oxidation of orthotoluenesulphamide".American Chemical Journal.1(6):426–439.Archivedfrom the original on 2021-02-24.Retrieved2022-02-24.From pages 430–431:
- "It possesses a 'very marked sweet taste, being much sweeter than cane-sugar'. The taste is perfectly pure. The minutest quantity of the substance, a bit of its powder scarcely visible, if placed upon the tip of the tongue, causes a sensation of pleasant sweetness throughout the entire cavity of the mouth. As stated above, the substance is soluble to only a slight extent in cold water, but if a few drops of the cold aqueous solution be placed in an ordinary goblet full of water, the latter then tastes like the sweetest syrup. Its presence can hence easily be detected in the dilutest solutions by the taste."
- ^US 319082,Fahlberg, C. & List, A., "Manufacture of saccharine compounds"
- ^Getman FH (1940).The Life of Ira Remsen.Easton, Pennsylvania: Journal of Chemical Education. p. 66.OCLC2640159.OL6411359M.
- ^"Sugar: A Cautionary Tale".FDA.gov.Archivedfrom the original on 2010-04-28.Retrieved2010-06-20.
- ^Dunn CW (1913).Federal, State, and Territorial Reference Manual of Pure Food and Drug Law: Dunn's Pure Food and Drug Legal Manual.p. 1327.Archivedfrom the original on 2017-03-13.
- ^"Monthly Bulletin".California State Board of Health. 1921. p. 21.Archivedfrom the original on 2017-03-14.
- ^abcdConis E (December 27, 2010)."Saccharin's mostly sweet following".Los Angeles Times.Archived fromthe originalon 2011-02-17.Retrieved2011-01-14.
- ^"Approved additives and E numbers | Food Standards Agency".food.gov.uk.Retrieved2024-05-02.
- ^"Saccharin cleared for use in foods in Canada".AgCanada.Health Canada.Archivedfrom the original on 2021-05-15.Retrieved2022-02-24.
- ^Takayama S, Sieber SM, Adamson RH, Thorgeirsson UP, Dalgard DW, Arnold LL, et al. (January 1998)."Long-term feeding of sodium saccharin to nonhuman primates: implications for urinary tract cancer".Journal of the National Cancer Institute.90(1):19–25.doi:10.1093/jnci/90.1.19.PMID9428778.
- ^"USPS Mailing Conditions".Archivedfrom the original on 2013-04-04.Retrieved2013-04-07.
- ^Sun M (28 December 2015)."Saccharin Delisted Effective April 6, 2001 as Known to the State to Cause Cancer".CA.gov.Archivedfrom the original on 10 March 2011.
- ^"Saccharin warning".Associated Press via Telegraph-Herald. 1973-05-22.Archivedfrom the original on 2021-12-23.Retrieved2011-06-09.
- ^Whysner J, Williams GM (1996). "Saccharin mechanistic data and risk assessment: urine composition, enhanced cell proliferation, and tumor promotion".Pharmacology & Therapeutics.71(1–2):225–52.doi:10.1016/0163-7258(96)00069-1.PMID8910956.
- ^Dybing E (December 2002). "Development and implementation of the IPCS conceptual framework for evaluating mode of action of chemical carcinogens".Toxicology.181–182:121–5.Bibcode:2002Toxgy.181..121D.doi:10.1016/S0300-483X(02)00266-4.PMID12505296.
- ^"Report on Carcinogens, Thirteenth Edition, Appendix B"(PDF).National Toxicology Program. pp.2–4. Archived fromthe original(PDF)on 2016-05-20.
Linked from"Saccharin index page".National Toxicology Program. November 18, 2014. Archived fromthe originalon 2016-03-07.Retrieved2016-02-29. - ^"Artificial Sweeteners and Cancer".National Cancer Institute. August 5, 2009. Archived fromthe originalon 2015-12-08.Retrieved2016-02-29.
- ^"EPA Removes Saccharin from Hazardous Substances Listing".December 14, 2010. Archived fromthe originalon 2010-12-24.Retrieved2011-01-14.
- ^Ager DJ, Pantaleone DP, Henderson SA,Katritzky AR,Prakash I, Walters DE (1998). "Commercial, Synthetic Nonnutritive Sweeteners".Angewandte Chemie International Edition.37(13–24):1802–1823.doi:10.1002/(SICI)1521-3773(19980803)37:13/14<1802::AID-ANIE1802>3.0.CO;2-9.
- ^Bungard G (1967). "Die Süßstoffe" [Sweeteners].Der Deutscher Apotheker.19:150.
- ^abLipinski GW. "Sweeteners".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a26_023.ISBN978-3-527-30673-2.
- ^Jaroentomeechai T, Yingsukkamol PK, Phurat C, Somsook E, Osotchan T, Ervithayasuporn V (November 2012). "Synthesis and reactivity of nitrogen nucleophiles-induced cage-rearrangement silsesquioxanes".Inorganic Chemistry.51(22):12266–72.doi:10.1021/ic3015145.PMID23134535.
- ^Ragnarsson U, Grehn L (May 2002). "Novel Gabriel reagents".Accounts of Chemical Research.24(10):285–289.doi:10.1021/ar00010a001.
- ^Sugasawa S, Abe K (1952)."A New Method for the Preparation of Secondary Amines. I. Synthesis of Aliphatic Secondary Amines".Yakugaku Zasshi.72(2):270–273.doi:10.1248/yakushi1947.72.2_270.Archivedfrom the original on 2017-10-03.
External links
edit- Media related toSaccharinat Wikimedia Commons